


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Synthesis of α-Amino Acids By modifying the nitrogen as a phthalimide salt |
|
|
|
Read More
Date: 25-7-2018
Date: 25-7-2018
Date: 13-12-2019
|
Synthesis of α-Amino Acids By modifying the nitrogen as a phthalimide salt
The propensity of amines to undergo multiple substitutions is removed, and a single clean substitution reaction of 1º- and many 2º-alkylhalides takes place. This procedure, known as the Gabriel synthesis, can be used to advantage in aminating bromomalonic esters, as shown in the upper equation of the following scheme. Since the phthalimide substituted malonic ester has an acidic hydrogen (colored orange), activated by the two ester groups, this intermediate may be converted to an ambident anion and alkylated. Finally, base catalyzed hydrolysis of the phthalimide moiety and the esters, followed by acidification and thermal decarboxylation, produces an amino acid and phthalic acid (not shown).
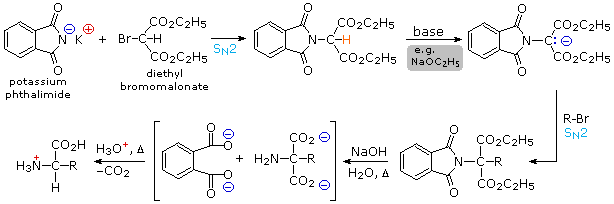



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|