


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 14-5-2017
Date: 14-5-2017
Date: 14-5-2017
|
CONDUCTORS
In some materials, electrons move easily from atom to atom. In others, the electrons move with difficulty. And in some materials, it is almost impossible to get them to move. An electrical conductor is a substance in which the electrons are highly mobile.
The best conductor, at least among common materials, at room temperature is pure elemental silver. Copper and aluminum are also excellent electrical conductors. Iron, steel, and various other metals are fair to good conductors of electricity. Some liquids are good conductors. Mercury is one example. Salt water is a fair conductor. Gases are, in general, poor conductors because the atoms or molecules are too far apart to allow a free exchange of electrons. However, if a gas becomes ionized, it can be a fair conductor of electricity.
Electrons in a conductor do not move in a steady stream like molecules of water through a garden hose. They pass from atom to atom (Fig. 1). This happens to countless atoms all the time. As a result, trillions of electrons pass a given point each second in a typical electric circuit.
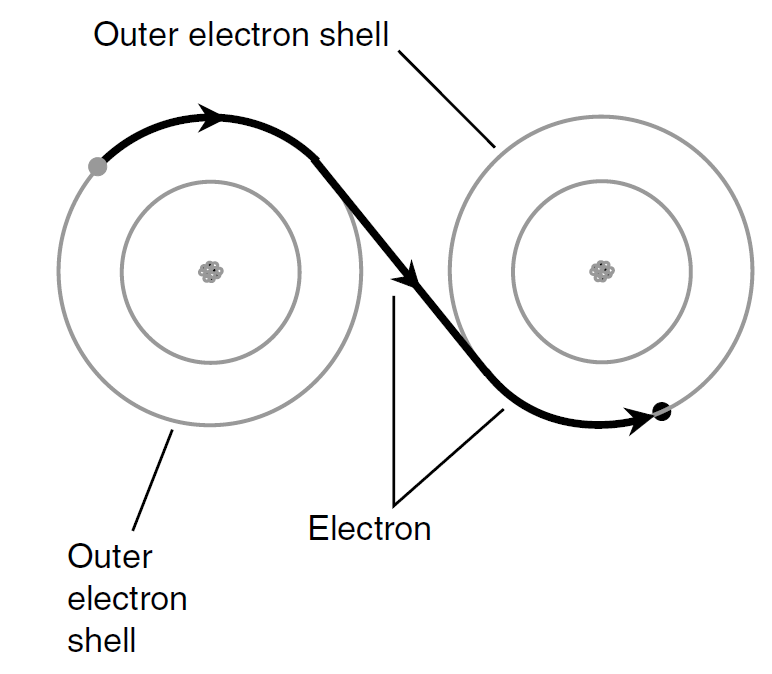
Fig.1. In an electrical conductor, electrons pass easily from atom to atom. This drawing is greatly simplified.
Imagine a long line of people, each one constantly passing a ball to his or her neighbor on the right. If there are plenty of balls all along the line, and if everyone keeps passing balls along as they come, the result is a steady stream of balls moving along the line. This represents a good conductor. If the people become tired or lazy and do not feel much like passing the balls along, the rate of flow decreases. The conductor is no longer very good.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
أولياء أمور الطلبة يشيدون بمبادرة العتبة العباسية بتكريم الأوائل في المراحل المنتهية
|
|
|