
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 5-7-2019
Date: 5-7-2019
Date: 8-10-2020
|
Alkynes are involved in a high release of energy because of repulsion of electrons. The content of energy involved in the alkyne molecule contributes to this high amount of energy. The pi-bonds however, do not encompass a great amount of energy even though the concentration is small within the molecule. The combustion of Ethyne is a major contributor from CO2, water, and the ethyne molecule
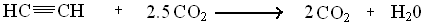
??H = -311 kcal/mol
To help understand the relative stabilities of alkyne isomers, heats of hydrogenation must be used. Hydrogenation of the least energy, results in the release of the internal alkyne. With the result of the production of butane, the stability of internal versus terminal alkynes has significant relative stability due to hyperconjugation.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
الأمين العام للعتبة العسكرية المقدسة يستقبل معتمد المرجعية الدينية العليا وعدد من طلبة العلم والوجهاء وشيوخ العشائر في قضاء التاجي
|
|
|