


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-1-2020
Date: 5-7-2019
Date: 8-1-2020
|
Hydration of Alkynes
Like alkenes alkynes can be hydrated by either of two methods. Direct addition of water catalyzed by mercury(II) ion yields the Markovnikov product, and indirect addition of water by a hydroboration–oxidation se≡uence yields the non-Markovnikov product.
Mercury(II)-Catalyzed Hydration of Alkynes
Alkynes don’t react directly with a≡ueous acid but will undergo hydration readily in the presence of mercury(II) sulfate as a Lewis acid catalyst. The reaction occurs with Markovnikov regiochemistry, so the -OH group adds to the more highly substituted carbon and the - H attaches to the less highly substituted one.
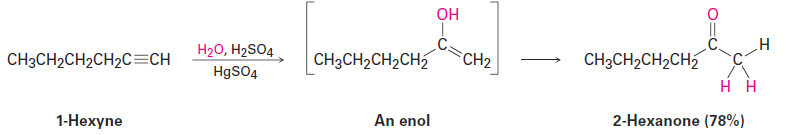
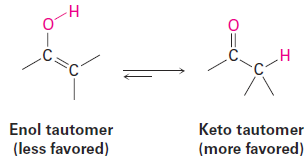
Interestingly, the actual product isolated from alkyne hydration is not a vinylic alcohol, or enol (ene + ol), but is instead a ketone. Although the enol is an intermediate in the reaction, it immediately rearranges into a ketone by a process called keto–enol tautomerism. The individual keto and enol forms are said to be tautomers, a word used to describe two isomers that undergo spontaneous interconversion accompanied by the change in position of a hydrogen. With few exceptions, the keto–enol tautomeric e≡uilibrium lies on the side of the ketone; enols are almost never isolated.
As shown in Figure 9-3, the mechanism of the mercury(II)-catalyzed alkyne hydration reaction is analogous to the oxymercuration reaction of alkenes. Electrophilic addition of mercury(II) ion to the alkyne gives a vinylic cation, which reacts with water and loses a proton to yield a mercury-containing enol intermediate. In contrast with alkene oxymercuration, however, no treatment with NaBH4 is necessary to remove the mercury. The acidic reaction conditions alone are sufficient to effect replacement of mercury by hydrogen. Tautomerization then gives the ketone.
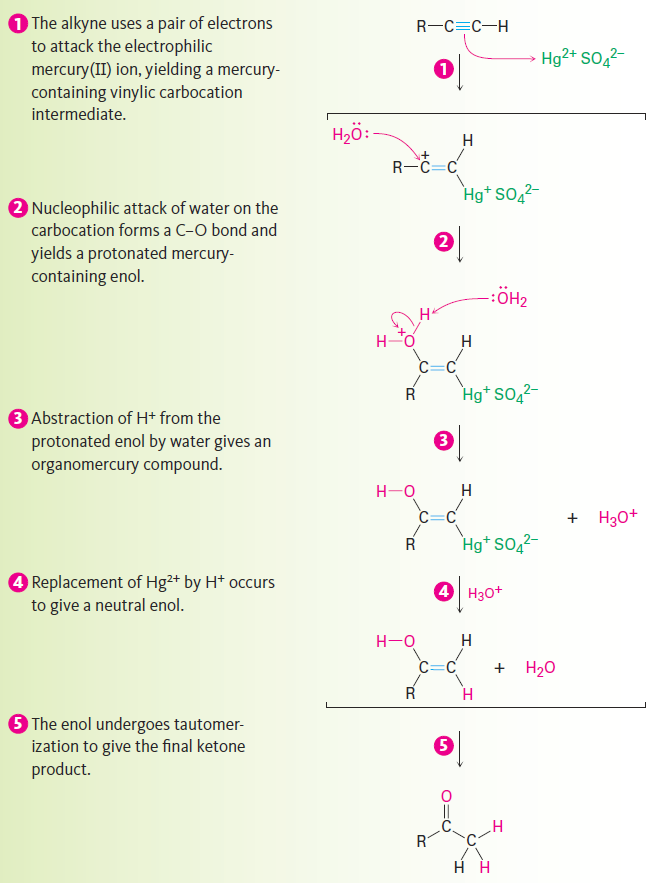
Figure 9-3: Mechanism of the mercury(II)-catalyzed hydration of an alkyne to yield a ketone. The reaction occurs through initial formation of an intermediate enol, which tautomerizes to the ketone.
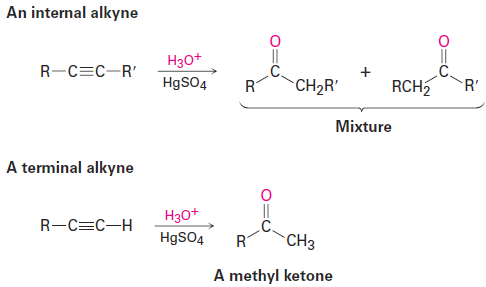
A mixture of both possible ketones results when an unsymmetrically substituted internal alkyne (RC≡CRꞌ( is hydrated. The reaction is therefore most useful when applied to a terminal alkyne (RC ≡ CH) because only a methyl ketone is formed.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|