
Standard potentials and spontaneity
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
149-151
الجزء والصفحة:
149-151
 2025-08-28
2025-08-28
 369
369
Standard potentials and spontaneity
Key point: A reaction is thermodynamically favourable (spontaneous) in the sense K>1, if E°>0, where E° is the difference of the standard potentials corresponding to the half-reactions into which the overall reaction may be divided. Thermodynamic arguments can be used to identify which reactions are spontaneous (that is, have a natural tendency to occur). The thermodynamic criterion of spontaneity is that, at constant temperature and pressure, the reaction Gibbs energy change, ∆rG, is negative. It is usually sufficient to consider the standard reaction Gibbs energy, ∆rG°, which is related to the equilibrium constant through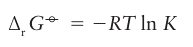 Anegative value of ∆r G° corresponds to K>1 and therefore to a ‘favourable’ reaction in the sense that the products dominate the reactants at equilibrium. It is important to realize, however, that ∆rG depends on the composition and that all reactions are spontaneous (that is, have ∆rG<0) under appropriate conditions. Because the overall chemical equation is the difference of two reduction half-reactions, the standard Gibbs energy of the overall reaction is the difference of the standard Gibbs energies of the two half-reactions. However, because reduction half-reactions always occur in pairs in any actual chemical reaction, only the difference in their standard Gibbs energies has any significance. Therefore, we can choose one half-reaction to have ∆rG° 0, and report all other values relative to it. By convention, the specially chosen half-reaction is the reduction of hydrogen ions:
Anegative value of ∆r G° corresponds to K>1 and therefore to a ‘favourable’ reaction in the sense that the products dominate the reactants at equilibrium. It is important to realize, however, that ∆rG depends on the composition and that all reactions are spontaneous (that is, have ∆rG<0) under appropriate conditions. Because the overall chemical equation is the difference of two reduction half-reactions, the standard Gibbs energy of the overall reaction is the difference of the standard Gibbs energies of the two half-reactions. However, because reduction half-reactions always occur in pairs in any actual chemical reaction, only the difference in their standard Gibbs energies has any significance. Therefore, we can choose one half-reaction to have ∆rG° 0, and report all other values relative to it. By convention, the specially chosen half-reaction is the reduction of hydrogen ions:
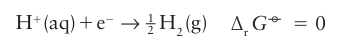
■ A brief illustration. The standard Gibbs energy for the reduction of Zn2 ions is found by determining experimentally that
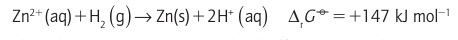 Then,because the H reduction half-reaction makes zero contribution to the reaction Gibbs energy (according to our convention), it follows that
Then,because the H reduction half-reaction makes zero contribution to the reaction Gibbs energy (according to our convention), it follows that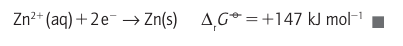 Standard reaction Gibbs energies may be measured by setting up a galvaniccell, an electrochemical cell in which a chemical reaction is used to generate an electric current, in which the reaction driving the electric current through the external circuit is the reaction of interest (Fig. 5.1). The potential difference between its electrodes is then measured. The cathode is the electrode at which reduction occurs and the anode is the site of oxidation. In practice, we must ensure that the cell is acting reversibly in a thermodynamic sense, which means that the potential difference must be measured with no current flowing. If desired, the measured potential difference can be converted to a reaction Gibbs energy by using ∆rG=-vfE, where v is the stoichiometric coefficient of the electrons transferred when the half-reactions are combined and F is Faraday’s constant (F=96.48 kC mol-1). Tabulated values, normally for standard conditions, are usually kept in the units in which they were measured, namely volts (V).
Standard reaction Gibbs energies may be measured by setting up a galvaniccell, an electrochemical cell in which a chemical reaction is used to generate an electric current, in which the reaction driving the electric current through the external circuit is the reaction of interest (Fig. 5.1). The potential difference between its electrodes is then measured. The cathode is the electrode at which reduction occurs and the anode is the site of oxidation. In practice, we must ensure that the cell is acting reversibly in a thermodynamic sense, which means that the potential difference must be measured with no current flowing. If desired, the measured potential difference can be converted to a reaction Gibbs energy by using ∆rG=-vfE, where v is the stoichiometric coefficient of the electrons transferred when the half-reactions are combined and F is Faraday’s constant (F=96.48 kC mol-1). Tabulated values, normally for standard conditions, are usually kept in the units in which they were measured, namely volts (V).
■ A brief comment. Standard conditions are all substances at 1 bar and unit activity. For reactions involving H+ ions, standard conditions correspond to Ph=0, approximately 1 M acid. Pure solids and liquids have unit activity. Although we use (nu) for the stoichiometric coefficient of the electron, electrochemical equations in inorganic chemistry are also commonly written with n in its place; we use to emphasize that it is a dimensionless number, not an amount in moles.
The potential that corresponds to the ∆rG° of a half-reaction is written E°, with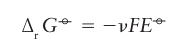 The potential E° is called the standard potential (or ‘standard reduction potential’, to emphasize that, by convention, the half-reaction is a reduction and written with the oxidized species and electrons on the left). Because ∆rG° for the reduction of H is arbitrarily set at zero, the standard potential of the H+/H2 couple is also zero at all temperatures:
The potential E° is called the standard potential (or ‘standard reduction potential’, to emphasize that, by convention, the half-reaction is a reduction and written with the oxidized species and electrons on the left). Because ∆rG° for the reduction of H is arbitrarily set at zero, the standard potential of the H+/H2 couple is also zero at all temperatures:
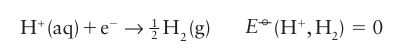 A brief illustration. For the Zn2+ /Zn couple, for which v=2, it follows from the measured value of ∆rG° that at 25C:
A brief illustration. For the Zn2+ /Zn couple, for which v=2, it follows from the measured value of ∆rG° that at 25C: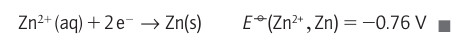 Because the standard reaction Gibbs energy is the difference of the ∆rG° values for the two contributing half-reactions, Ecell° for an overall reaction is also the difference of the two standard potentials of the reduction half-reactions into which the overall reaction can be divided. Thus, from the half-reactions given above it follows that the difference is
Because the standard reaction Gibbs energy is the difference of the ∆rG° values for the two contributing half-reactions, Ecell° for an overall reaction is also the difference of the two standard potentials of the reduction half-reactions into which the overall reaction can be divided. Thus, from the half-reactions given above it follows that the difference is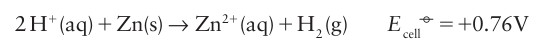 Note that the E° values for couples (and their half-reactions) are called standard potentials and that their difference is denoted Ecell° and called the standard cell potential. The consequence of the negative sign in eqn 5.2 is that a reaction is favourable (in the sense K>1) if the corresponding standard cell potential is positive. Because E°>0 for the reaction in the illustration (E°= +0.76 V), we know that zinc has a thermodynamic tendency to reduce H ions under standard conditions (aqueous, pH=0, and Zn+2 at unit activity); that is zinc metal dissolves in acids. The same is true for any metal that has a couple with a negative standard potential.
Note that the E° values for couples (and their half-reactions) are called standard potentials and that their difference is denoted Ecell° and called the standard cell potential. The consequence of the negative sign in eqn 5.2 is that a reaction is favourable (in the sense K>1) if the corresponding standard cell potential is positive. Because E°>0 for the reaction in the illustration (E°= +0.76 V), we know that zinc has a thermodynamic tendency to reduce H ions under standard conditions (aqueous, pH=0, and Zn+2 at unit activity); that is zinc metal dissolves in acids. The same is true for any metal that has a couple with a negative standard potential.
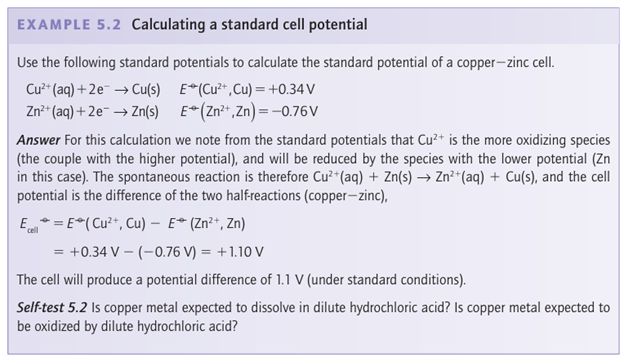
Combustion is a familiar type of redox reaction, and the energy that is released can be exploited in heat engines. A fuel cell converts a chemical fuel directly into electrical power (Box 5.1).
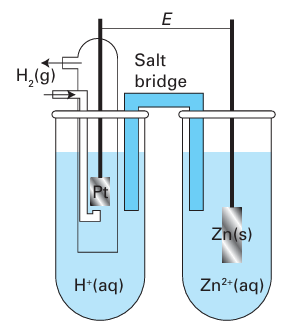
Fig. 5.1 A schematic diagram of a galvanic cell. The standard potential, Ecell°, is the potential difference when the cell is not generating current and all the substances are in their standard states.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


