
Acidic and neutral solvents
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
142
الجزء والصفحة:
142
 2025-08-28
2025-08-28
 344
344
Acidic and neutral solvents
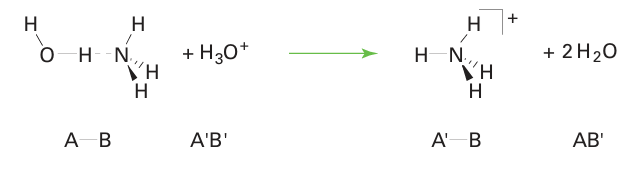
Key points: Hydrogen bond formation is an example of Lewis complex formation; other solvents may also show Lewis acid character. Hydrogen bonding (Section 10.6) can be regarded as an example of complex formation. The ‘reaction’ is between A–H (the Lewis acid) and: B (the Lewis base) and gives the com plex conventionally denoted A–H ... B. Hence, many solutes that form hydrogen bonds with a solvent can be regarded as dissolving because of complex formation. A consequence of this view is that an acidic solvent molecule is displaced when proton transfer occurs:
Liquid sulfur dioxide is a good soft acidic solvent for dissolving the soft base benzene. Unsaturated hydrocarbons may act as acids or bases by using their π or π* orbitals as frontier orbitals. Alkanes with electronegative substituents, such as haloalkanes (for example, CHCl3), are significantly acidic at the hydrogen atom. Saturated fluorocarbon solvents lack Lewis acid and base properties
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


