
Interpretation of hardness
 المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
المؤلف:
Peter Atkins, Tina Overton, Jonathan Rourke, Mark Weller, and Fraser Armstrong
 المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
المصدر:
Shriver and Atkins Inorganic Chemistry ,5th E
 الجزء والصفحة:
ص139-140
الجزء والصفحة:
ص139-140
 2025-08-27
2025-08-27
 390
390
Interpretation of hardness
Key points: Hard acid–base interactions are predominantly electrostatic; soft acid–base interactions are predominantly covalent.
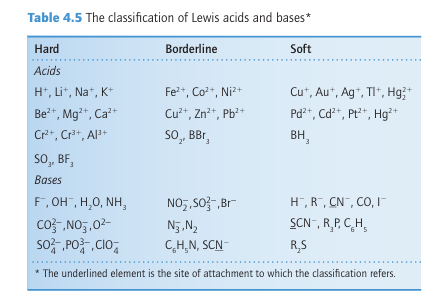
The bonding between hard acids and bases can be described approximately in terms of ionic or dipole–dipole interactions. Soft acids and bases are more polarizable than hard acids and bases, so the acid–base interaction has a more pronounced covalent character. Although the type of bond formation is a major reason for the distinction between the two classes, there are other contributions to the Gibbs energy of complex formation and hence to the equilibrium constant. Important factors are: 1) Competition with the solvent in reactions in solution. 2) The rearrangement of the substituents of the acid and base that may be necessary to permit formation of the complex. 3) Steric repulsion between substituents on the acid and the base. Any of these contributions can have a marked effect on the outcome of a reaction. It is important to note that although we associate soft acid/soft base interactions with covalent bonding, the bond itself may be surprisingly weak. This point is illustrated by reactions involving Hg2, a representative soft acid. The metathesis reaction
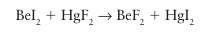
is exothermic, as predicted by the hard–soft rule. The bond dissociation energies (in kilo joules per mole) measured for these molecules in the gas phase are.
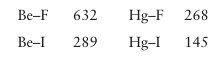
Therefore, it is not the large Hg–I bond energy that ensures that the reaction is exothermic but the especially strong bond between Be and F, which is an example of a hard–hard inter action. In fact an Hg atom forms only weak bonds to any other atom. In aqueous solution, the reason why Hg2 forms a much more stable complex with iodide ions compared to chloride ions is the much more favourable hydration energy of Cl–.
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


