
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 13-4-2017
Date: 26-7-2016
Date: 12-4-2017
|
Amino Acids Can Act as Acids and Bases
When an amino acid is dissolved in water, it exists in solution as the dipolar ion, or zwitterion (German for “hybrid ion”), shown in Figure 3–9. A zwitterion can act as either an acid (proton donor):
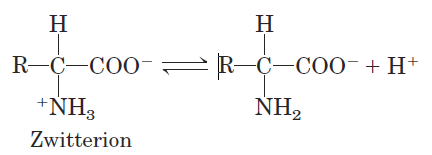
or a base (proton acceptor):
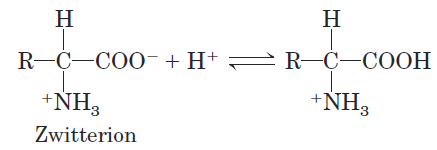
Substances having this dual nature are amphoteric and are often called ampholytes (from “amphoteric electrolytes”). A simple monoamino monocarboxylic α-amino acid, such as alanine, is a diprotic acid when fully protonated—it has two groups, the —COOH group and the—NH3 _ group, that can yield protons:
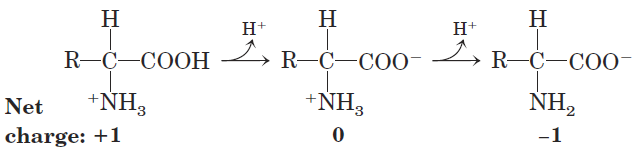



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
ملاكات العتبة العباسية المقدسة تُنهي أعمال غسل حرم مرقد أبي الفضل العباس (عليه السلام) وفرشه
|
|
|