


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-9-2016
Date: 26-7-2016
Date: 25-7-2016
|
Molarity: Comparing solute to solution
Molarity is the concentration unit chemists use most often, because it utilizes moles. The mole concept is central to chemistry, and molarity lets chemists easily work solutions into reaction stoichiometry.
Molarity (M) is defined as the moles of solute per liter of solution. Mathematically, it looks like this:
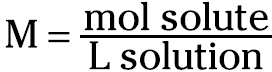
For example, you can take 1 mole (abbreviated as mol) of KCl (formula weight of 74.55 g/mol; see Chapter 10) and dissolve and dilute the 74.55 grams to 1 liter of solution in a volumetric flask. You then have a 1-molar solution of KCl. You can label that solution as 1 M KCl.
When preparing molar solutions, always dissolve and dilute to the required volume. So to dissolve 74.55 grams of KCl to 1 liter of solution, you don’t add the 74.55 grams to 1 liter of water. You want to end up with a final volume of 1 liter.
Here’s another example: If 25.0 grams of KCl are dissolved and diluted to 350.0 milliliters, how would you calculate the molarity of the solution? You know that molarity is moles of solute per liter of solution. So you can take the grams, convert them to moles using the formula weight of KCl (74.55 g/mol), and divide them by 0.350 liters (350.0 milliliters). You can set up the equation like this:
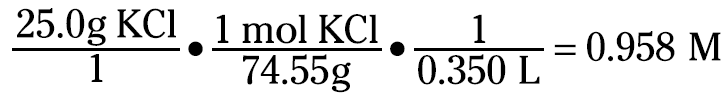
Now suppose that you want to prepare 2.00 liters of a 0.550 M KCl solution. The first thing you do is calculate how much KCl you need to weigh:
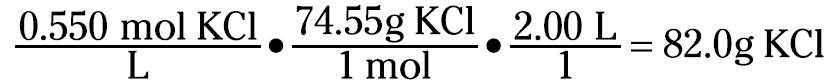
You then take that 82.0 grams of KCl and dissolve and dilute it to 2.00 liters.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
سماحة السيد الصافي يؤكد ضرورة تعريف المجتمعات بأهمية مبادئ أهل البيت (عليهم السلام) في إيجاد حلول للمشاكل الاجتماعية
|
|
|