


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 7-8-2016
Date: 21-8-2016
Date: 13-7-2016
|
Leaky Balloon
Sometimes helium gas in a low-temperature physics lab is kept temporarily in a large rubber bag at essentially atmospheric pressure. A physicist left a 40-L bag filled with He floating near the ceiling before leaving on vacation. When she returned, all the helium was gone (diffused through the walls of the bag). Find the entropy change of the gas. Assume that the atmospheric helium concentration is approximately 5 ×10-4%. What is the minimum work needed to collect the helium back into the bag?
SOLUTION
Let us consider the bag as part of a very large system (the atmosphere) which initially has N molecules of air, which we consider as one gas, and molecules N1 of helium. The bag has volume V0, and the number of helium molecules is N0. Omitting all the temperature-dependent terms, we may write for the initial entropy of the system
 (1)
(1)
When the helium has diffused out, we have
 (2)
(2)
We wish to find  in the limit where V >> V0. Then
in the limit where V >> V0. Then
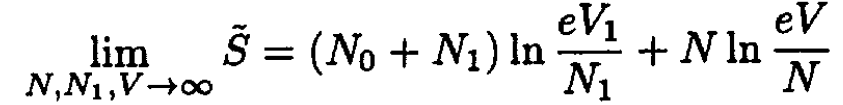 (3)
(3)
We then obtain
 (4)
(4)
where n0/n1 ~ 1/(5 × 10-6) is the concentration ratio of helium molecules in the bag to their concentration in the air. In regular units
 (5)
(5)
Substituting the standard pressure and temperature into (5) gives
 (6)
(6)
The minimum work necessary to separate the helium at constant temperature is
 (7)
(7)
∆S1 = -∆S, since after we separate the helium molecules from the rest of the air, the total entropy of that system would decrease. So




|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
المجمع العلميّ يُواصل عقد جلسات تعليميّة في فنون الإقراء لطلبة العلوم الدينيّة في النجف الأشرف
|
|
|