

علم الكيمياء

تاريخ الكيمياء والعلماء المشاهير

التحاضير والتجارب الكيميائية

المخاطر والوقاية في الكيمياء

اخرى

مقالات متنوعة في علم الكيمياء

كيمياء عامة


الكيمياء التحليلية

مواضيع عامة في الكيمياء التحليلية

التحليل النوعي والكمي

التحليل الآلي (الطيفي)

طرق الفصل والتنقية


الكيمياء الحياتية

مواضيع عامة في الكيمياء الحياتية

الكاربوهيدرات

الاحماض الامينية والبروتينات

الانزيمات

الدهون

الاحماض النووية

الفيتامينات والمرافقات الانزيمية

الهرمونات


الكيمياء العضوية

مواضيع عامة في الكيمياء العضوية

الهايدروكاربونات

المركبات الوسطية وميكانيكيات التفاعلات العضوية

التشخيص العضوي

تجارب وتفاعلات في الكيمياء العضوية


الكيمياء الفيزيائية

مواضيع عامة في الكيمياء الفيزيائية

الكيمياء الحرارية

حركية التفاعلات الكيميائية

الكيمياء الكهربائية


الكيمياء اللاعضوية

مواضيع عامة في الكيمياء اللاعضوية

الجدول الدوري وخواص العناصر

نظريات التآصر الكيميائي

كيمياء العناصر الانتقالية ومركباتها المعقدة


مواضيع اخرى في الكيمياء

كيمياء النانو

الكيمياء السريرية

الكيمياء الطبية والدوائية

كيمياء الاغذية والنواتج الطبيعية

الكيمياء الجنائية


الكيمياء الصناعية

البترو كيمياويات

الكيمياء الخضراء

كيمياء البيئة

كيمياء البوليمرات

مواضيع عامة في الكيمياء الصناعية

الكيمياء الاشعاعية والنووية
Substitution Reaction
المؤلف:
Charge DistributionWilliam Reusch
المصدر:
Virtual Textbook of Organic Chemistry
الجزء والصفحة:
............
28-7-2018
1994
Substitution reaction
An early method of preparing phenol (the Dow process) involved the reaction of chlorobenzene with a concentrated sodium hydroxide solution at temperatures above 350 ºC. The chief products are phenol and diphenyl ether (see below). This apparent nucleophilic substitution reaction is surprising, since aryl halides are generally incapable of reacting by either an SN1 or SN2 pathway.
C6H5–Cl + NaOH solution |
350 ºC |
C6H5–OH + C6H5–O–C6H5 + NaCl |
The presence of electron-withdrawing groups (such as nitro) ortho and para to the chlorine substantially enhance the rate of substitution, as shown in the set of equations presented on the left below. To explain this, a third mechanism for nucleophilic substitution has been proposed. This two-step mechanism is characterized by initial addition of the nucleophile (hydroxide ion or water) to the aromatic ring, followed by loss of a halide anion from the negatively charged intermediate. This is illustrated by clicking the "Show Mechanism" button next to the diagram. The sites over which the negative charge is delocalized are colored blue, and the ability of nitro, and other electron withdrawing, groups to stabilize adjacent negative charge accounts for their rate enhancing influence at the ortho and para locations.
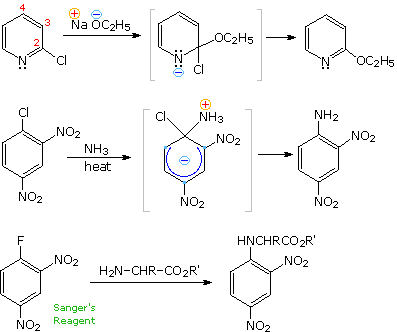
Three additional examples of aryl halide nucleophilic substitution are presented on the right. Only the 2- and 4-chloropyridine isomers undergo rapid substitution, the 3-chloro isomer is relatively unreactive. Nitrogen nucleophiles will also react, as evidenced by the use of Sanger's reagent for the derivatization of amino acids. The resulting N-2,4-dinitrophenyl derivatives are bright yellow crystalline compounds that facilitated analysis of peptides and proteins, a subject for which Frederick Sanger received one of his two Nobel Prizes in chemistry.
 |
Additional Examples
|
|---|
Such addition-elimination processes generally occur at sp2 or sp hybridized carbon atoms, in contrast to SN1 and SN2 reactions. When applied to aromatic halides, as in the present discussion, this mechanism is called SNAr. Some distinguishing features of the three common nucleophilic substitution mechanisms are summarized in the following table.
|
Mechanism |
Number of Steps |
Bond Formation Timing |
Carbon Hybridization |
|---|---|---|---|
|
SN1 |
Two |
After Bond Breaking |
Usually sp3 |
|
SN2 |
One |
Simultaneous with |
Usually sp3 |
|
SNAr |
Two |
Prior to Bond Breaking |
Usually sp2 |
 الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
الاكثر قراءة في مواضيع عامة في الكيمياء العضوية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام) قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)
قسم الشؤون الفكرية يصدر مجموعة قصصية بعنوان (قلوب بلا مأوى)


















