
The Origins of the Interatomic Force
 المؤلف:
Roger J Blin-Stoyle, FRS
المؤلف:
Roger J Blin-Stoyle, FRS
 المصدر:
Physics of Particles, Matter and the Universe
المصدر:
Physics of Particles, Matter and the Universe
 الجزء والصفحة:
p 85
الجزء والصفحة:
p 85
 24-5-2016
24-5-2016
 2837
2837
The Origins of the Interatomic Force
The nature of the force between two atoms. Broadly speaking it is a force that is attractive when two atoms come close to each other and then eventually becomes repulsive when they become very close. In the last chapter the essential structure of an atom was outlined: a very small but massive positively charged nucleus surrounded by electrons in different quantum states. For a given atom, these states are filled with the requisite number of electrons according to the limitations of the Pauli exclusion principle. In an atom the innermost electrons are in filled ‘shells’ (i.e. in stable sets of quantum states, which, by the exclusion principle, cannot accommodate any more electrons) and usually there are a few electrons in unfilled outer shells. When two atoms approach each other electrical forces clearly come into operation and these, together with quantum effects, determine the detailed nature of the force between two atoms. The precise explanation of the force depends, as might be expected, on the details of the atomic structure and there are essentially three types of mechanism in operation. These will now be outlined.
Ionic Force. This force is the simplest to understand and accounts, for example, for the binding of sodium to chlorine in common salt. Sodium is an atom with 11 electrons, ten of which are in filled shells, and one is fairly loose. Chlorine, on the other hand, has 17 electrons and is just one short of having completely filled shells it has a ‘hole’ in the shell. When these two atoms approach each other it is easy for the spare sodium electron to jump over into the hole and the resultant system is much more stable, but the sodium atom, having lost a negatively charged electron, has a net positive charge, whilst the chlorine atom, having gained an electron, has a net negative charge. We have, therefore, a positive sodium ion close to a negative chlorine ion and clearly there will be an attractive electric force between them due to their opposite charges and it is this which holds them together in the molecule (see figure 1.1(a)). If they move very close together the electrons in the filled shells start encroaching on each other‘s orbits. This is forbidden by the exclusion principle and so repulsion begins to take over. In addition there is also an increasingly important repulsive force due to the close approach of the positively charged atomic nuclei. Hence the general features of the interatomic force are produced. The joining together of two atoms by the ionic force is referred to as ionic bonding and manifests itself in many diatomic molecules.
Covalent Force. The ionic force occurs because an electron is handed over from one atom to the other. Covalent forces, on the other hand, result from the ‘sharing’ of electrons between each atom.
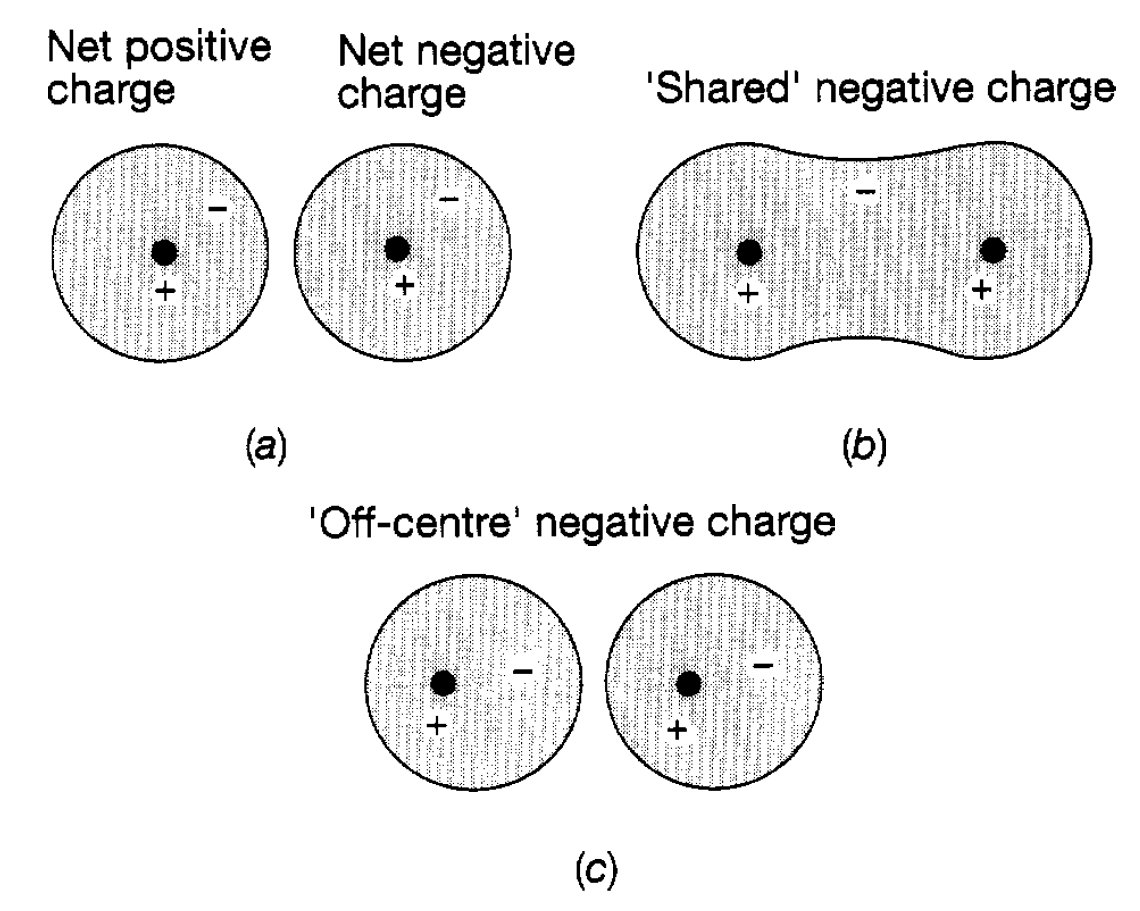
Figure 1.1 : Charge distributions relevant to interatomic forces: (a) ionic; (b) covalent; (c) Van der Waals.
To understand this the simplest system to consider is the hydrogen molecule, consisting of two hydrogen atoms held together by the covalent force. Each hydrogen atom has a single electron and, as the atoms approach each other, the electron of one begins to feel the electrical attraction due to the positive nucleus of the other. At a close enough distance the electrons move across between the atoms and partially occupy each other’s quantum states the electrons are shared between the two atoms. It will be recollected that the lowest state of the hydrogen atom can only accommodate two electrons (with opposite spins) and therefore, to some extent, the state is being filled. This makes for a very stable structure. The two electrons tend to spend most of their time between the two nuclei (see figure 1.l(b)) and the resultant central negative charge draws the two positively charged nuclei together. Of course, repulsion begins to set in when the two nuclei become very close together because of their like charges. Covalent forces play a major role in the formation of more complex molecules and the joining together of atoms in this way is referred to as covalent bonding. When an atom has several electrons outside filled shells this, in turn, enables covalent bonds to be formed with several other atoms and this mechanism underlies the formation of a wide spread of molecular structures from water (two hydrogen atoms and one oxygen atom) through to the immense complexity of DNA.
Van der Waals Force. This is a much weaker force than the two just discussed and does not involve any transfer of electrons between the two interacting atoms. It is, for example, the origin of the force between inert gas atoms (atoms which only have filled shells of electrons). In such atoms the electrons move around in their quantum states and although on average the electron charge is distributed symmetrically about the nuclear charge it is sometimes ‘off centre’. For example in figure 1.l(c) the average negative electron charge in the left hand atom is displaced slightly to the right of the nucleus. Since this charge is now nearer to the right hand atom than the (positive) nuclear charge, there is a net pull on the nucleus of the right hand atom and a slight push on its electrons, leading to a corresponding off centre distribution of charge in that atom. As a result of this lopsidedness of the two charge distributions there is small attractive force between the two atoms, but the force is not strong enough to hold such atoms together as a molecule and this is why they are referred to as inert. However, this force plays an important role in explaining intermolecular forces, particularly when the molecules under consideration have an intrinsic lopsidedness because they consist of two or more different types of atom. It is in terms of these different interatomic forces that we understand and explain the properties of the many varieties of molecule and also the cohesion of atoms in the various forms of matter. As far as molecules and their behaviour are concerned this is largely the province of chemistry and little more will be said about them except to note that their ‘bulk’ motions are also quantized. By bulk motions is meant motions of their component atoms. For example, in a diatomic molecule (think of it as being like a dumb bell) the whole molecule can rotate with angular momentum quantized in units of h and with corresponding quantized energy levels. Similarly the two atoms can vibrate about their equilibrium separation with resultant vibrational energy levels of the type. As a result, in addition to electrons in molecules making transitions from one energy state to another as in atoms, there can also be transitions between the rotational and vibrational energy levels, all leading to the emission or absorption of electromagnetic radiation. Molecular spectra are, therefore, much more complicated in their structure than atomic spectra, particularly for molecules containing three or even more atoms. Properties of the three states of matter were explained in terms of the interatomic and intermolecular forces just discussed. We now extend this discussion further and seek explanations and understanding of the electromagnetic properties of matter.
 الاكثر قراءة في الفيزياء الكيميائية
الاكثر قراءة في الفيزياء الكيميائية
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


