


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 18-4-2016
Date: 11-4-2016
Date: 20-3-2016
|
(LASING MEDIUM (EXCIMER LASERS
Energy levels in an excimer laser are defined by the state of the atomic components. When unbound, the energy of the system depends purely on the separation between the individual atoms; as the atoms move closer together, energy rises. This is illustrated by the lower curve in Figure 1.1. The lower energy level in an excimer system is defined as a separation of the halogen and inert gas atoms. This is the normal state for an inert species such as argon, krypton, or xenon, which will not, under ordinary circumstances, form compounds with any other atom. The upper

Figure 1.1. Excimer energy levels.
energy state is formed when the inert atom and the halogen form an excited dimer (or excimer) molecule. This is a temporary state that forms only for an instant in time when the excited atoms combine to form a molecule. The energy of the excimer molecule is much higher than that of the unbound individual atoms and also depends on the interatomic spacing in a similar manner to that of any other molecule, such as hydrogen. As with the hydrogen molecule example, numerous vibrational levels are possible. Collectively, these closely spaced levels form a band serving as the upper lasing level.
The laser tube is filled with a mixture of an inert gas and a halogen such as fluorine or chlorine. When an enormous, fast-rising electric current (generated by a mechanism similar to that of a nitrogen laser) passes through the mixture, gas atoms become extremely excited and excimer molecules form. This forms the ULL for the species, which has a short lifetime, on the order of tens of nanoseconds. In this respect the excimer laser resembles that of a nitrogen laser and a fast discharge is required to generate the excimer molecules effectively and ensure an inversion before the lifetime of the species has elapsed.
Various excimer species are outlined in Table 1.1, which also lists the relative power output relative to KrF, the lost powerful excimer laser. Although KrF produces the most powerful output, other gas mixtures, such as XeCl, are popular for use in excimer lasers. Shortcomings of the KrF laser include the output wavelength of 249 nm, which is absorbed readily by air, and the extremely corrosive nature of fluorine, which shortens the useful life of the gas mix in this laser. Worse yet from a beam-management perspective is ArF, which produces a wavelength so short that it produces ozone gas from atmospheric oxygen as it passes through air. When using ArF, beam paths must be enclosed and flushed with dry nitrogen, helium, or argon. XeCl, on the other hand, has a longer wavelength, allowing better transmission in air and the use of considerably cheaper optics. The gas mixture also has a much longer useful lifetime (up to 10 times longer, by some estimates). The useful lifetime of lasing gases may also be extended by using a cryogenic gas processor in which the lasing gas mixture is passed through coils immersed in liquid nitrogen to trap impurities (some of which adsorb from internal components and others produced by the hostile environment inside the laser) in the gas mixture. A cryogenic gas processor such as that shown in Figure 1.2 is a standard option on most commercial excimer lasers. In the figure two copper lines, evident on the top of the processor, connect to the laser vessel itself, and laser gas is recirculated continually by a pump in the processor
TABLE 1.1. Excimer Species.
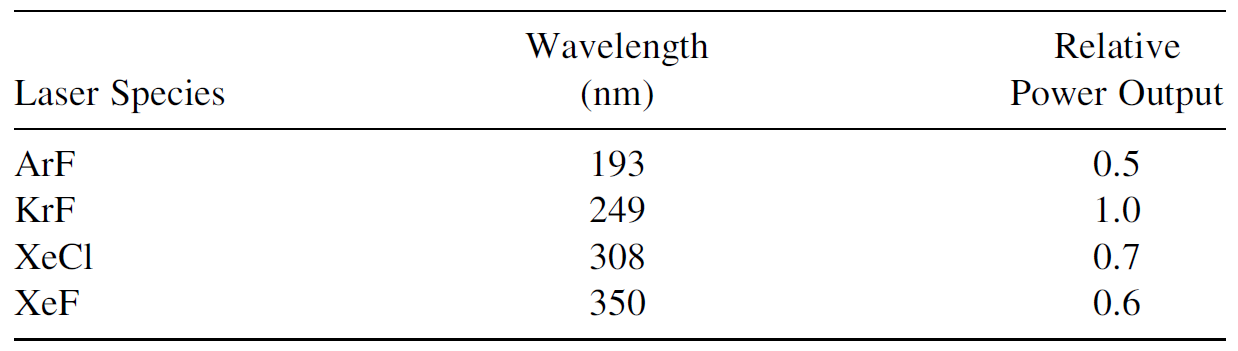

Figure 1.2. Cryogenic gas processor.
through these lines and into the processor’s cold trap. Many large excimer lasers (i.e., intended for prolonged use) have gas ports allowing connection of a gas processor. The cost of a gas fill is quite pricey, so gas processors pay back quickly for many industrial users.
The actual gas mixture employed consists of a small quantity of fluorine combined with a moderate amount of inert gas, with the balance helium or neon. Pure fluorine is such a corrosive and toxic gas to handle that it is not available in pure form but only in diluted form, as 5% fluorine and 95% neon gas (or possibly, helium). Typical tube pressures are around 2 to 3 atm. Table 1.2 lists typical
TABLE 1.2. Excimer Gas Mixtures
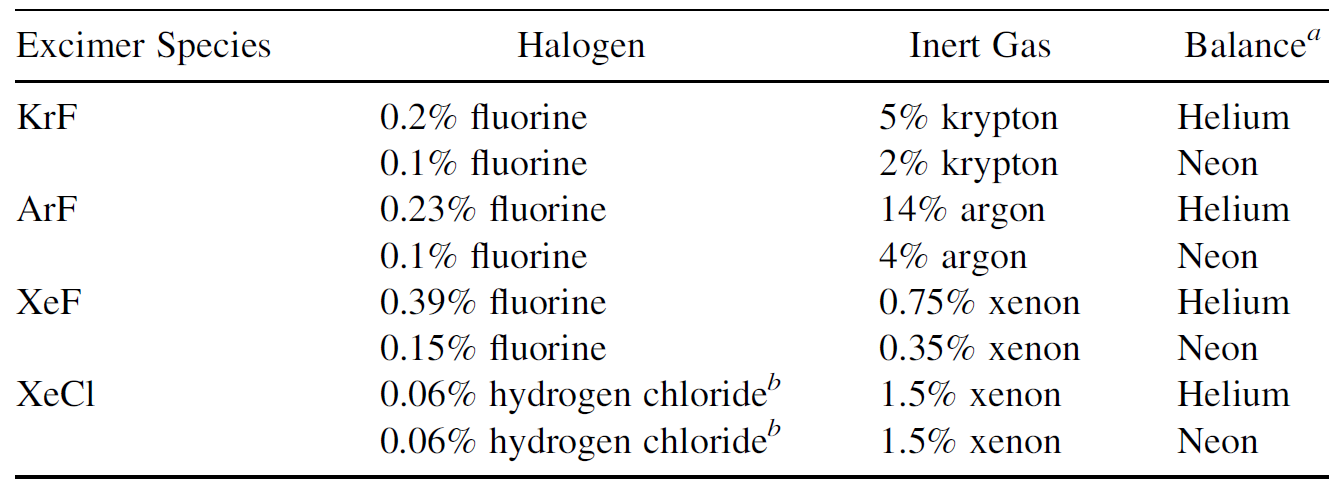
excimer gas mixtures for two different excimer lasers, as specified by a manufacturer of such lasers. In all cases, only a tiny amount of halogen (fluorine or HCl) is used in the gas mixture, along with a moderate amount of inert gas, with the vast majority of the mixture an inert buffer gas such as helium or neon. Aside from assisting in excitation of the molecule and helping to distribute the discharge, helium also helps to conduct heat away from the discharge so that the gas mixture can be cooled by a heat exchanger inside the laser housing.
_______________________________________________________________
a The use of neon as a buffer gas does not altogether eliminate the need for helium, since helium is used, along with a small percentage of fluorine, to passivate (condition the surface of ) system components in the laser.
b In both cases, hydrogen gas is present in the supply cylinder at about one-half the concentration of hydrogen chloride to prolong gas life. Even so, halogen gas shelf life is only about six to nine months.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
اتحاد كليات الطب الملكية البريطانية يشيد بالمستوى العلمي لطلبة جامعة العميد وبيئتها التعليمية
|
|
|