


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 6-11-2015
Date: 7-12-2015
Date: 8-11-2015
|
Immunological Tolerance
T-cell tolerance, as defined by a lack of immune reactivity can be due to a number of processes: Firstly, Negative selection in the thymus (referred to as deletion); secondly a simple lack of reactivity to antigen (self or nonself) as a result of the antigen having not been present in the secondary lymphoid organs in a sufficient quantity or for a sufficient amount of time; and thirdly an excessive stimulation of T-cells resulting from the ubiquitous presence of sufficient antigen resulting in T cell exhaustion. Finally, it may also be possible that T cells can become temporarily “anergized” by partial or incomplete antigen stimulation. As a general rule, self-reactive (autoimmune) B cells are not generally deleted by negative selection and can therefore be present in the periphery. Exceptions to this rule include B cells specific for membrane-bound self-determinants, some of which are deleted or anergized. B cells react promptly to antigens, even self-antigens, which are arranged repetitively. However, they only react to soluble monomeric antigens if they additionally receive T cell help. Thus, B-cell non-reactivity largely results from a lack of patterned antigen presentation structures or as a result of T-cell tolerance.
Immunological tolerance describes the concept that the immune system does not normally react to autologous structures, but maintains the ability to react against foreign antigens. Tolerance is acquired, and can be measured as the selective absence of immunological reactivity against specified antigens.
T-Cell Tolerance
A distinction can be made between central tolerance, which develops in the thymus and is based on the negative selection (deletion) of T cells recognizing self antigens present in the thymus, and peripheral tolerance. Peripheral tolerance results in the same outcome as central tolerance, however, this form of tolerance involves antigen recognition byantigen-reactive peripheral T cells, followed by a process of clonal cell proliferation, end differentiation and death. The following mechanisms have been postulated, and in some cases confirmed, to account for a lack of peripheral T-cell responsiveness:
-T-cell indifference or ignorance. Both host and foreign antigens present only within peripheral epithelial, mesenchymal or neuroectodermal cells and tissues—and which do not migrate, or are not transported by APCs, in sufficient amounts to the organized lymphoid organs—are simply ignored by T and B cells. Most self-antigens, not present in the serum or in lymphohematopoietic cells, belong to this category and are ignored despite the fact that they are potentially immunogenic. Certain viruses, and their antigens, actually take advantage of this system of ignorance. For instance, the immune system ignores the rabies virus when it is restricted to axons, and papilloma viruses as long as the antigens are restricted to keratinocytes (warts). The main reason why many self antigens, and some foreign antigens, are ignored by T cells is that immune responses can only be induced within the spleen or in lymph nodes, and non-activated (or naive) T cells do not migrate into the periphery. It has also been postulated that those naive T and B cells which do encounter antigens in the periphery will become anergized, or inactivated, due to a lack of the so-called costimulatory or secondary signals at these sites. However, the evidence supporting this theory is still indirect. Experiments seeking to understand the “indifference” of T cells are summarized in the box on p. 92f. In all probability, a great many self-antigens (as well as peripheral tumors) are ignored by the immune system in this way. These self-antigens represent a potential target for autoimmunity.
-Complete, exhaustive T-cell induction. When an antigen, self or non-self, enters a lymphoid organ it encounters many APCs and T cells, resulting in the extremely efficient activation those T cells carrying the appropriate TCR. During such a scenario the responding T cells differentiate into shortlived effector cells which only survive for two to four days. This induction phase may actually correspond to the postulated phenomenon of anergy. Should this be the case, anergy—defined as the inability of T cells to react to antigen stimulation in vitro—may in fact be explained by the responding cells having already entered a pathway of cell death (apoptosis). Once all the terminally differentiated effector T cells have died, immune reactivity against the stimulating antigen ends. Tolerance is hereafter maintained, as should the responsible antigen have entered into the thymus those newly maturing thymocytes will be subjected to the process of negative selection (e.g., as seen in chronic systemic (viremic) infections with noncytopathic viruses). Moreover, those newly matured T cells which may have escaped negative selection and emigrated into the periphery will continuously be induced to undergo activation and exhaustion within the secondary lymphoid organs.
Exhaustive T-cell induction most likely occurs in responses to hepatitis C virus and HIV, and has been observed in mice experimentally infected with the noncytopathic virus causing lymphocytic choriomeningitis. Successful establishment of lymphocyte chimerism following liver transplants appears to based on the same principle. For example, a relatively short period of immunosuppression following transplantation may allow the establishment of numerous dendritic cells from the transplanted organ within the secondary lymphoid organs of the recipient, resulting in the subsequent elimination of those recipient T cells which react against the foreign MHC molecules.
In summary, the non-responsiveness of T-cells can be achieved by: negative selection in the thymus; by excessive induction in the periphery; or by sequestration of the antigen outside the lymphoid organs. Persistence of the antigen within the lymphoid tissues is a prerequisite for the first two mechanisms. For the third mechanism, it is the absence of antigen within lymphatic organs which guarantees non-responsiveness. There is also a necessary role for 'second'- or 'costimulatory'-signals in the activation of T cells within lymphoid tissues, however, their role in T-cell responsiveness within solid organs remains unclear.
B-Cell Tolerance
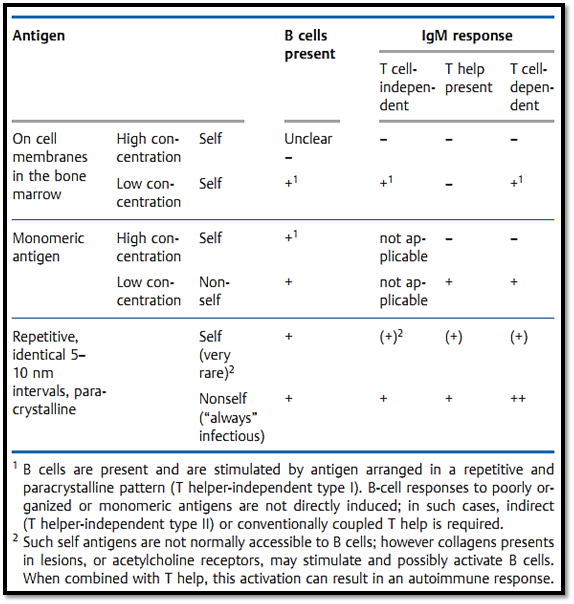
In contrast to classic central T-cell tolerance, B cells capable of recognizing self-antigens appear unlikely to be subjected to negative selection (Table 1). B-cell regeneration in the bone marrow is a very intensive process, during which antigen selection probably does not play an important role. Although negative selection of bone marrow B cells can be demonstrated experimentally for highly-expressed membrane-bound MHC molecules (in antibody-transgenic mice)—this apparently does not occur for more rare membrane-bound antigens, or for most soluble self-antigens. As a general rule, these potentially self-reactive B cells are not stimulated to produce an immune response because the necessary T helper cells are not present as a result of having being subjected to negative selection in the thymus. B cell and antibody tolerance is therefore largely a result of T cell tolerance which results in the absence of T help.
The finding that a certain antigenic structures and sequences can activate B cells in the absence of T help indicates that autoreactive B cells which are present could be prompted to produce an IgM autoantibody response via Ig cross-linking by paracrystalline multimeric antigens. However, since selfantigens are not normally accessible to B cells in such repetitive paracrystal- line patterns, the induction of IgM autoantibody responses is not normally observed. It is interesting to note that DNA and collagen, which often contribute to chronic autoantibody responses, exhibit repetitive antigen structures. These structures become accessible to B cells within inflamed lesions, and may therefore induce autoantibody responses in certain circumstances. A chronic autoantibody response of the IgG type, however, always requires T help arising from the presentation of self-peptides by MHC class II molecules. Ignored self-peptides, and in all likelihood infectious agents, may play a role in providing such T help. (For instance Klebsiella or Yersinia in rheumatic diseases, Coxsackie virus infections in diabetes, or other chronic parasitic infections.)
Table 1 B Cells Do Not Differentiate between Self and Nonself Antigens, but Rather Distinguish Repetitive (Usually Nonself) from Monomeric )Usually Self) Antigens
References
Zinkernagel, R. M. (2005). Medical Microbiology.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|