


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
أقرأ أيضاً
التاريخ: 31-8-2017
التاريخ: 27-1-2016
التاريخ: 15-2-2016
التاريخ: 27-1-2016
|
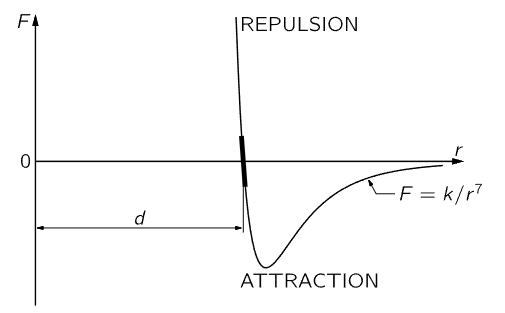
Fig. 12–2. The force between two atoms as a function of their distance of separation.
We shall next discuss the characteristics of molecular forces. These are forces between the atoms, and are the ultimate origin of friction. Molecular forces have never been satisfactorily explained on a basis of classical physics; it takes quantum mechanics to understand them fully. Empirically, however, the force between atoms is illustrated schematically in Fig. 12–2, where the force F between two atoms is plotted as a function of the distance r between them. There are different cases: in the water molecule, for example, the negative charges sit more on the oxygen, and the mean positions of the negative charges and of the positive charges are not at the same point; consequently, another molecule nearby feels a relatively large force, which is called a dipole–dipole force. However, for many systems the charges are very much better balanced, in particular for oxygen gas, which is perfectly symmetrical. In this case, although the minus charges and the plus charges are dispersed over the molecule, the distribution is such that the center of the minus charges and the center of the plus charges coincide. A molecule where the centers do not coincide is called a polar molecule, and charge times the separation between centers is called the dipole moment. A nonpolar molecule is one in which the centers of the charges coincide. For all nonpolar molecules, in which all the electrical forces are neutralized, it nevertheless turns out that the force at very large distances is an attraction and varies inversely as the seventh power of the distance, or F=k/r7, where k is a constant that depends on the molecules. Why this is we shall learn only when we learn quantum mechanics. When there are dipoles the forces are greater. When atoms or molecules get too close they repel with a very large repulsion; that is what keeps us from falling through the floor!
These molecular forces can be demonstrated in a fairly direct way: one of these is the friction experiment with a sliding glass tumbler; another is to take two very carefully ground and lapped surfaces which are very accurately flat, so that the surfaces can be brought very close together. An example of such surfaces is the Johansson blocks that are used in machine shops as standards for making accurate length measurements. If one such block is slid over another very carefully and the upper one is lifted, the other one will adhere and also be lifted by the molecular forces, exemplifying the direct attraction between the atoms on one block for the atoms on the other block.
Nevertheless, these molecular forces of attraction are still not fundamental in the sense that gravitation is fundamental; they are due to the vastly complex interactions of all the electrons and nuclei in one molecule with all the electrons and nuclei in another. Any simple–looking formula we get represents a summation of complications, so we still have not got the fundamental phenomena.
Since the molecular forces attract at large distances and repel at short distances, as shown in Fig. 12–2, we can make up solids in which all the atoms are held together by their attractions and held apart by the repulsion that sets in when they are too close together. At a certain distance d (where the graph in Fig. 12–2 crosses the axis) the forces are zero, which means that they are all balanced, so that the molecules stay that distance apart from one another. If the molecules are pushed closer together than the distance d they all show a repulsion, represented by the portion of the graph above the r–axis. To push the molecules only slightly closer together requires a great force, because the molecular repulsion rapidly becomes very great at distances less than d. If the molecules are pulled slightly apart there is a slight attraction, which increases as the separation increases. If they are pulled sufficiently hard, they will separate permanently—the bond is broken.
If the molecules are pushed only a very small distance closer, or pulled only a very small distance farther than d, the corresponding distance along the curve of Fig. 12–2 is also very small, and can then be approximated by a straight line. Therefore, in many circumstances, if the displacement is not too great the force is proportional to the displacement. This principle is known as Hooke’s law, or the law of elasticity, which says that the force in a body which tries to restore the body to its original condition when it is distorted is proportional to the distortion. This law, of course, holds true only if the distortion is relatively small; when it gets too large the body will be torn apart or crushed, depending on the kind of distortion. The amount of force for which Hooke’s law is valid depends upon the material; for instance, for dough or putty the force is very small, but for steel it is relatively large. Hooke’s law can be nicely demonstrated with a long coil spring, made of steel and suspended vertically. A suitable weight hung on the lower end of the spring produces a tiny twist throughout the length of the wire, which results in a small vertical deflection in each turn and adds up to a large displacement if there are many turns. If the total elongation produced, say, by a 100–gram weight, is measured, it is found that additional weights of 100 grams will each produce an additional elongation that is very nearly equal to the stretch that was measured for the first 100 grams. This constant ratio of force to displacement begins to change when the spring is overloaded, i.e., Hooke’s law no longer holds.



|
|
|
|
لمكافحة الاكتئاب.. عليك بالمشي يوميا هذه المسافة
|
|
|
|
|
|
|
تحذيرات من ثوران بركاني هائل قد يفاجئ العالم قريبا
|
|
|
|
|
|
|
العتبة العباسية تشارك في معرض النجف الأشرف الدولي للتسوق الشامل
|
|
|