


 الفيزياء الكلاسيكية
الفيزياء الكلاسيكية
 الكهربائية والمغناطيسية
الكهربائية والمغناطيسية
 علم البصريات
علم البصريات
 الفيزياء الحديثة
الفيزياء الحديثة
 النظرية النسبية
النظرية النسبية
 الفيزياء النووية
الفيزياء النووية
 فيزياء الحالة الصلبة
فيزياء الحالة الصلبة
 الليزر
الليزر
 علم الفلك
علم الفلك
 المجموعة الشمسية
المجموعة الشمسية
 الطاقة البديلة
الطاقة البديلة
 الفيزياء والعلوم الأخرى
الفيزياء والعلوم الأخرى
 مواضيع عامة في الفيزياء
مواضيع عامة في الفيزياء|
Read More
Date: 31-12-2020
Date: 17-9-2020
Date: 31-12-2020
|
NOT JUST A MIXTURE!
A compound is not the same thing as a mixture of elements. Sometimes, however, when elements are mixed (and, if necessary, given a jolt of energy), compounds result because the elements undergo chemical reactions with each other. If hydrogen and oxygen are mixed, the result is a colorless, odorless gas. A spark will cause the molecules to join together to form water vapor. This reaction will liberate energy in the form of light and heat. Under the right conditions, there will be an explosion because the two elements join eagerly. When atoms of elements join together to form a compound, the resulting particles are molecules. Figure 1 is a simplified diagram of a water molecule.
Compounds often, but not always, appear different from any of the elements that make them up. At room temperature and pressure, both hydrogen and oxygen are gases. But water under the same conditions is a liquid. The heat of the reaction just described, if done in the real world, would result in water vapor initially, and water vapor is a colorless, odorless gas. However, some of this vapor would condense into liquid water if the temperature got
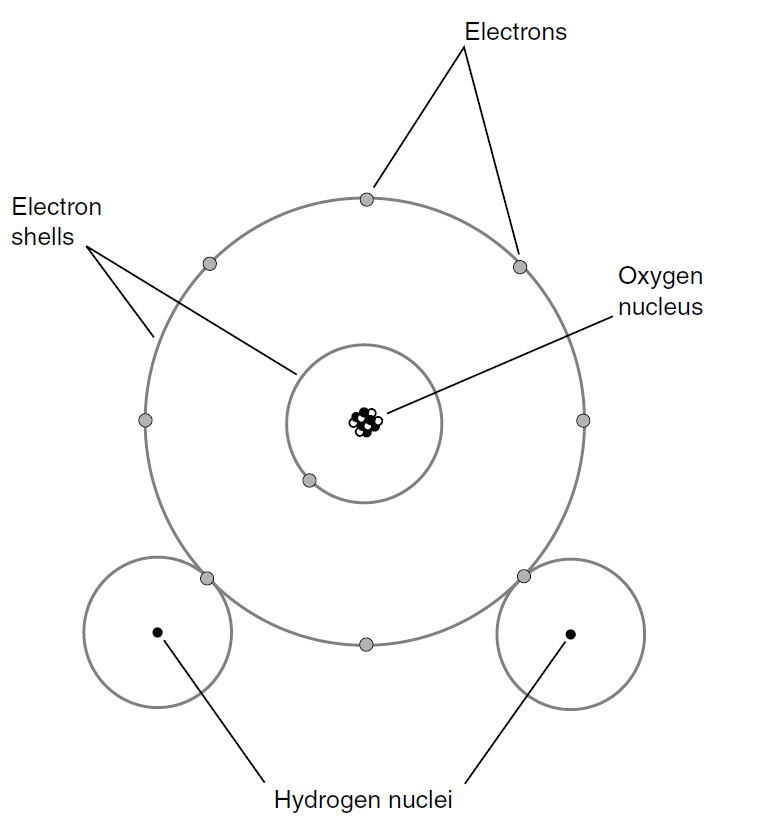
Fig. 1. Simplified diagram of a water molecule.
low enough for dew to form. Some of it would become solid, forming frost, snow, or ice if the temperature dropped below the freezing point of water.
A note of caution: Do not try an experiment like this! You could be severely burned. In the extreme, if enough of the hydrogen-oxygen air is inhaled, your lungs will be injured to the point where you may die of asphyxiation. We sometimes read or hear news reports about home experimenters who blew themselves up with chemistry sets. Don’t become the subject matter for one of these stories!
Another common example of a compound is rust. This forms when iron joins with oxygen. Iron is a dull gray solid, and oxygen is a gas; however, iron rust is a maroon-red or brownish powder, completely unlike either of the elements from which it is formed. The reaction between iron and oxygen takes place slowly, unlike the rapid combination of hydrogen and oxygen when ignited. The rate of the iron-oxygen reaction can be sped up by the presence of water, as anyone who lives in a humid climate knows.



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
مؤسسة وارث الخيرية التابعة للعتبة الحسينية تفتح أبوابها لطلبة الجامعات للتطوع في مرافقة الأطفال المصابين بالسرطان خلال رحلتهم العلاجية
|
|
|