


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 28-10-2020
Date: 17-9-2019
Date: 9-7-2019
|
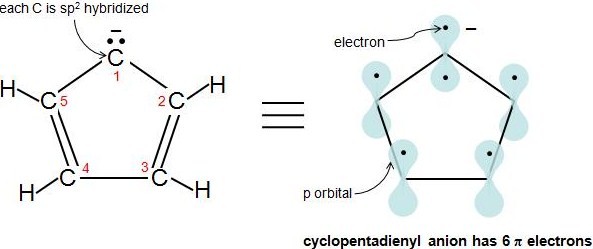
Hückel's Rule also applies to ions. As long as a compound has 4n+2 π electrons, it does not matter if the molecule is neutral or has a charge. For example, cyclopentadienyl anion is an aromatic ion. How do we know that it is fully conjugated? That is, how do we know that each atom in this molecule has 1 p orbital? Let's look at the following figure. Carbons 2-5 are sp2 hybridized because they have 3 attached atoms and have no lone electron pairs. What about carbon 1? Another simple rule to determine if an atom is sp2 hybridized is if an atom has 1 or more lone pairs and is attached to an sp2 hybridized atom, then that atom is sp2 hybridized also. Therefore, carbon 1 has a p orbital. Cyclopentadienyl anion has 6 π electrons and fulfills the 4n+2 rule.
.jpg?revision=1&size=bestfit&width=411&height=172)



|
|
|
|
للعاملين في الليل.. حيلة صحية تجنبكم خطر هذا النوع من العمل
|
|
|
|
|
|
|
"ناسا" تحتفي برائد الفضاء السوفياتي يوري غاغارين
|
|
|
|
|
|
|
المجمع العلمي يقيم ورشة تطويرية ودورة قرآنية في النجف والديوانية
|
|
|