


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 7-9-2018
Date: 2-12-2019
Date: 16-7-2019
|
Oxygen assumes only two oxidation states in its organic compounds (–1 in peroxides and –2 in other compounds). Sulfur, on the other hand, is found in oxidation states ranging from –2 to +6, as shown in the following table (some simple inorganic compounds are displayed in orange).
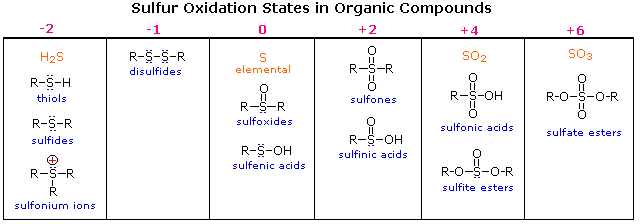
Try drawing Lewis-structures for the sulfur atoms in these compounds. If you restrict your formulas to valence shell electron octets, most of the higher oxidation states will have formal charge separation, as in equation 2 above. The formulas written here neutralize this charge separation by double bonding that expands the valence octet of sulfur. Indeed, the S=O double bonds do not consist of the customary σ & π-orbitals found in carbon double bonds. As a third row element, sulfur has five empty 3d-orbitals that may be used for p-d bonding in a fashion similar to p-p (π) bonding. In this way sulfur may expand an argon-like valence shell octet by two (e.g. sulfoxides) or four (e.g. sulfones) electrons. Sulfoxides have a fixed pyramidal shape (the sulfur non-bonding electron pair occupies one corner of a tetrahedron with sulfur at the center). Consequently, sulfoxides having two different alkyl or aryl substituents are chiral. Enantiomeric sulfoxides are stable and may be isolated.
Thiols also differ dramatically from alcohols in their oxidation chemistry. Oxidation of 1º and 2º-alcohols to aldehydes and ketones changes the oxidation state of carbon but not oxygen. Oxidation of thiols and other sulfur compounds changes the oxidation state of sulfur rather than carbon. We see some representative sulfur oxidations in the following examples. In the first case, mild oxidation converts thiols to disufides. An equivalent oxidation of alcohols to peroxides is not normally observed. The reasons for this different behavior are not hard to identify. The S–S single bond is nearly twice as strong as the O–O bond in peroxides, and the O–H bond is more than 25 kcal/mole stronger than an S–H bond. Thus, thermodynamics favors disulfide formation over peroxide.
Mild oxidation of disufides with chlorine gives alkylsulfenyl chlorides, but more vigorous oxidation forms sulfonic acids (2nd example). Finally, oxidation of sulfides with hydrogen peroxide (or peracids) leads first to sulfoxides and then to sulfones.
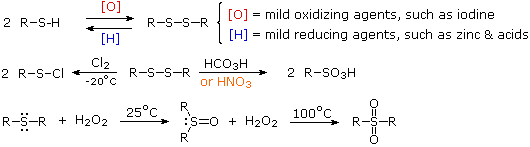
The nomenclature of sulfur compounds is generally straightforward. The prefix thio denotes replacement of a functional oxygen by sulfur. Thus, -SH is a thiol and C=S a thione. The prefix thia denotes replacement of a carbon atom in a chain or ring by sulfur, although a single ether-like sulfur is usually named as a sulfide. For example, C2H5SC3H7 is ethyl propyl sulfide and C2H5SCH2SC3H7 may be named 3,5-dithiaoctane. Sulfonates are sulfonate acid esters and sultones are the equivalent of lactones. Other names are noted in the table above.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|