
آخر المواضيع المضافة


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-10-2020
Date: 3-8-2019
Date: 5-9-2018
|
The difference in oxidation states between nitrogen and phosphorus is less pronounced than between oxygen and sulfur. Organophosphorus compounds having phosphorus oxidation states ranging from –3 to +5, as shown in the following table, are well known (some simple inorganic compounds are displayed in green). As in the case of sulfur, the P=O double bonds drawn in some of the formulas do not consist of the customary sigma & pi-orbitals found in carbon double bonds. Phosphorus is a third row element, and has five empty 2d-orbitals that may be used for p-d bonding in a fashion similar to p-p (π) bonding. In this way phosphorus may expand an argon-like valence shell octet by two electrons (e.g. phosphine oxides).
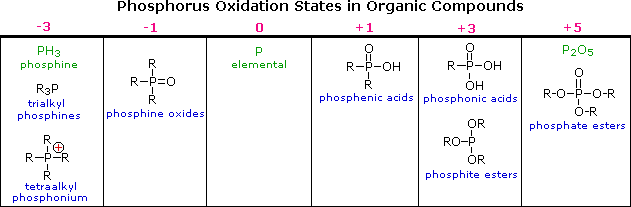



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|