


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 11-9-2016
Date: 12-4-2017
Date: 26-7-2016
|
van der Waals Interactions Are Weak Interatomic Attractions
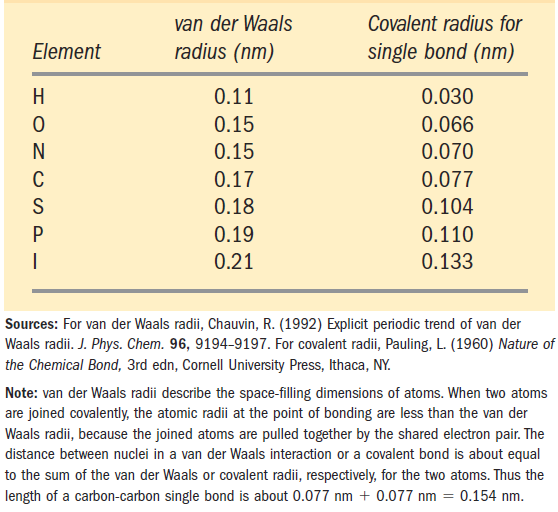
When two uncharged atoms are brought very close together, their surrounding electron clouds influence each other. Random variations in the positions of the electrons around one nucleus may create a transient electric dipole, which induces a transient, opposite electric dipole in the nearby atom. The two dipoles weakly attract each other, bringing the two nuclei closer. These weak attractions are called van der Waals interactions. As the two nuclei draw closer together, their electron clouds begin to repel each other. At the point where the van der Waals attraction exactly balances this repulsive force, the nuclei are said to be in van der Waals contact. Each atom has a characteristic van der Waals radius, a measure of how close that atom will allow another to approach (Table 1.1). In the “space-filling” molecular models shown throughout this book, the atoms are depicted in sizes proportional to their van der Waals radii.
TABLE 1.1 van der Waals Radii and Covalent (Single-Bond) Radii of Some Elements



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
العتبة العباسية المقدسة تقدم دعوة إلى جامعة البصرة التقنية الجنوبية للمشاركة في الحفل المركزي لتخرج طلبة الجامعات
|
|
|