

 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 14-9-2016
Date: 28-11-2016
Date: 28-11-2016
|
oxidation states in aqueous solution
In the preceding sections, we have, with some degree of success, attempted to rationalize irregular trends in some thermodynamic properties of the first row d-block metals. Now we consider the variation in Eo values for equilibrium 1.1 the more negative the value of Eo, the less easily M2+ is reduced.
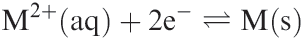 (1.1)
(1.1)
This turns out to be a difficult problem. Water is relatively easily oxidized or reduced, and the range of oxidation states on which measurements can be made under aqueous conditions is therefore restricted, e.g. Sc(II) and Ti(II) would liberate H2. Values of Eo(M2+/M) are related to energy changes accompanying the processes:
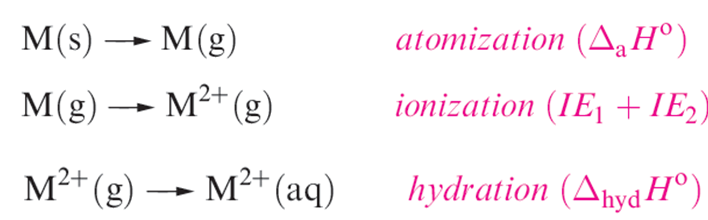
In crossing the first row of the d-block, the general trend is for ΔhydHo to become more negative. There is also a successive increase in the sum of the first two ionization energies albeit with discontinuities at Cr and Cu (Figure 1.1).
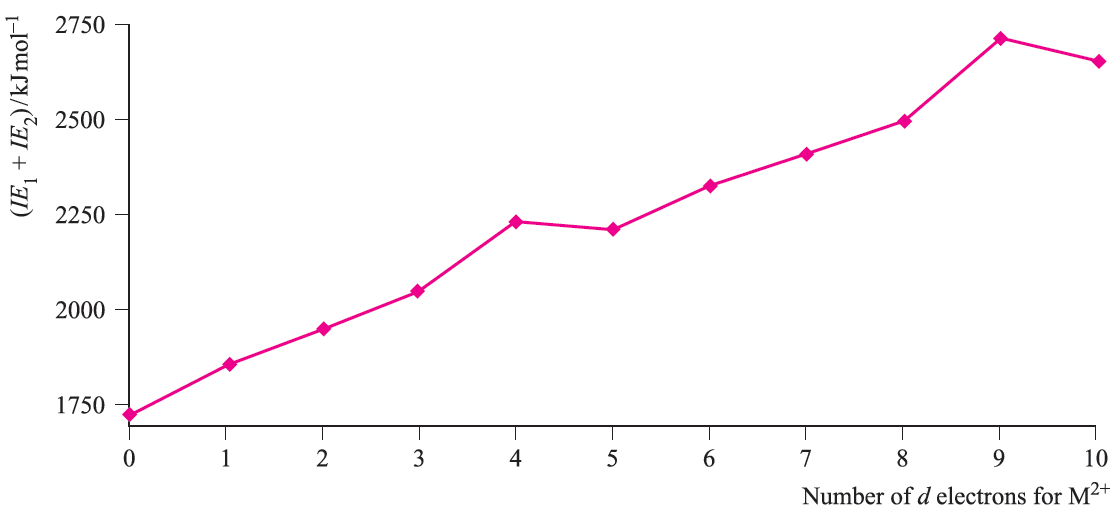
Fig. 1.1 The variation in the sum of the first and second ionization energies as a function of d n configuration for the first row metals; the point for d0 corresponds to M = Ca.
Values of ΔaHo vary erratically and over a wide range with a particularly low value for zinc (Table 5.2). The net effect of all these factors is an irregular variation in values of Eo)M2+/M) across the row, and it is clearly not worthwhile discussing the relatively small variations in LFSEs. Consider now the variations in Eo(M3+/M2+) across the row. The enthalpy of atomization is no longer relevant and we are concerned only with trends in the third ionization energy (Table 1.1) and the hydration energies of M2+ and M3+. Experimental values for Eo(M3+/M2+) (Table 1.1) are restricted to the middle of the series; Sc(II) and Ti(II) would reduce water while Ni(III), Cu(III) and Zn(III) would oxidize it. In general, larger values of IE3 correspond to more positive Eo values; this suggests that a steady increase in the difference between the hydration energies of M3+ and M2+ (which would become larger as the ions become smaller) is outweighed by the variation in IE3. The only pair of metals for which the change in Eo appears out of step is vanadium and chromium.
Table 1.1 Standard reduction potentials for the equilibrium M3ً+(aq)+ e- ⇋ M2+ً(aq) and values of the third ionization energies.

The value of IE3 for Cr is 165 kJ mol-1 greater than for V and so it is harder to oxidize gaseous Cr2+ than V2+. In aqueous solution however, Cr2+ is a more powerful reducing agent than V2+. These oxidations correspond to changes in electronic configuration of d3 → d2 for V and d4 → d3 for Cr. The V2+, V3+, Cr2+ and Cr3+ hexaaqua ions are high-spin; oxidation of V2+ is accompanied by a loss of LFSE while there is a gain in LFSE (i.e. more negative) upon oxidation of Cr2+ (minor consequences of the Jahn–Teller effect are ignored). Using values of Δoct from Table 20.2, these changes in LFSE are expressed as follows:
Change in LFSE on oxidation of V2+ is
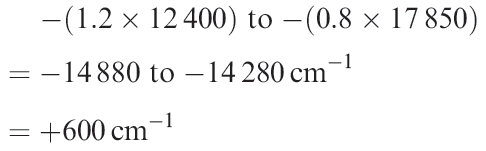
Change in LFSE on oxidation of Cr2+ is

The gain in LFSE upon formation of Cr3+ corresponds to ≈150 kJ mol-1 and largely cancels out the effect of the third ionization energy. Thus, the apparent anomaly of Eo(Cr3+/Cr2+) can be mostly accounted for in terms of LFSE effects – a considerable achievement in view of the simplicity of the theory.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|