
Heat Loss
 المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
 المصدر:
A GUIDE TO PHYSICS PROBLEMS
المصدر:
A GUIDE TO PHYSICS PROBLEMS
 الجزء والصفحة:
part 2 , p 8
الجزء والصفحة:
part 2 , p 8
 29-8-2016
29-8-2016
 1726
1726
Heat Loss
An immersion heater of power J = 500 W is used to heat water in a bowl. After 2 minutes, the temperature increases from T1 = 85oC to T2 = 90°C. The heater is then switched off for an additional minute, and the temperature drops by ∆T = 1oC. Estimate the mass of the water in the bowl. The thermal capacity of water c = 4.2 × 103 J/kg K.
SOLUTION
Let τ1 = 2 min be the time the heater is operating. The energy added to the water and bowl will heat the water as well as the environment. We will assume that the heat loss Q1 to the surroundings is proportional to the elapsed time τ1 and that the changing temperature difference 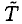 between the water and room temperature T ~ 23oC. During this phase, we may write
between the water and room temperature T ~ 23oC. During this phase, we may write
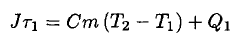 (1)
(1)
The heat loss Q1 is actually a time integral of some proportionality constant λ times the temperature difference as the water heats up. However, 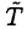 only varies by 5oC out of an average 65oC, so we will ignore the variation. The heat loss during the second phase is given by
only varies by 5oC out of an average 65oC, so we will ignore the variation. The heat loss during the second phase is given by
 (2)
(2)
Just as in the heating phase, the heat loss is proportional to the elapsed time τ2 = τ1/2. Since 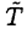 again only changes a little, we have
again only changes a little, we have
 (3)
(3)
We may now eliminate Q1 from(1), yielding

 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة


