
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Entropy of Ideal Gas
المؤلف:
Sidney B. Cahn, Gerald D. Mahan And Boris E. Nadgorny
المصدر:
A GUIDE TO PHYSICS PROBLEMS
الجزء والصفحة:
part 2 , p 20
26-8-2016
2096
Entropy of Ideal Gas
A vessel of volume V1 contains N molecules of an ideal gas held at temperature τ and pressure P1. The energy of a molecule may be written in the form

where εk denotes the energy levels corresponding to the internal states of the molecules of the gas.
a) Evaluate the free energy F. Explicitly display the dependence on the volume V1.
Now consider another vessel, also at temperature τ, containing the same number of molecules of the identical gas held at pressure P2.
b) Give an expression for the total entropy of the two gases in terms of P1, P2, τ, N.
c) The vessels are then connected to permit the gases to mix without doing work. Evaluate explicitly the change in entropy of the system. Check whether your answer makes sense by considering the special case V1 = V2 (P1 = P2).
SOLUTION
a) For an ideal gas the partition function factors; however, we must take the sum of N identical molecules divided by the number of interchanges N! to account for the fact that one microscopic quantum state corresponds to a number of different points in phase space. So
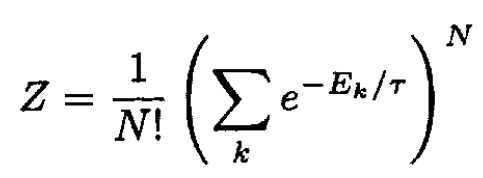 (1)
(1)
Now, the Helmholtz free energy, F, is given by
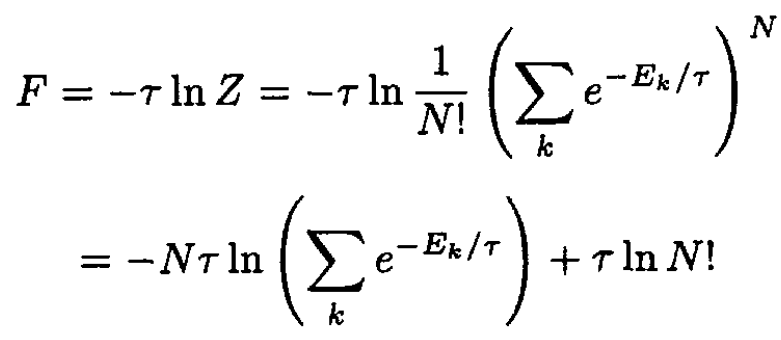 (2)
(2)
Using Stirling’s formula, ln we obtain
we obtain
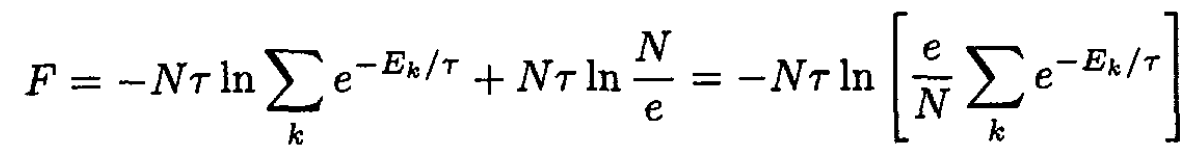 (3)
(3)
Using the explicit expression for the molecular energy Ek, we can rewrite (S.4.38.3) in the form
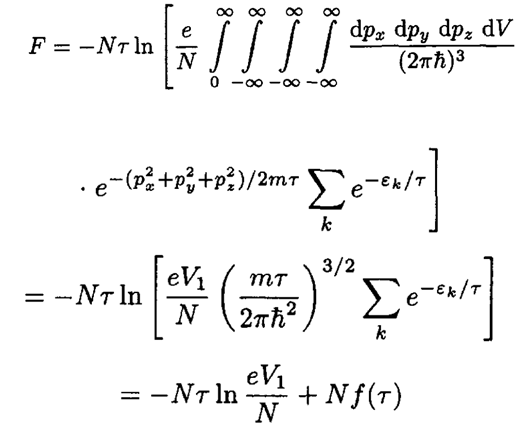 (4)
(4)
Here we used the fact that the sum depends only on temperature, so we
can define f(τ):
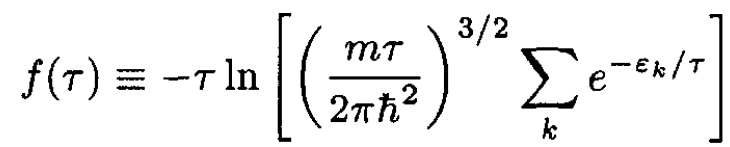 (5)
(5)
b) Now we can calculate the total entropy S of the two gases (it is important that the gases be identical so that f(τ) is the same for both vessels):
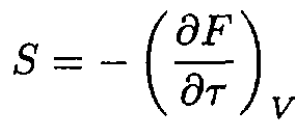 (6)
(6)
where F is defined by (4).
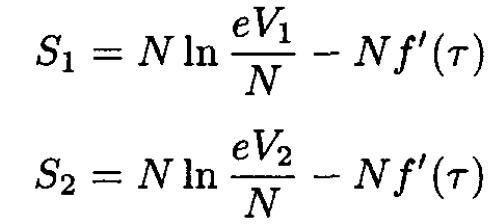 (7)
(7)
We have for total entropy
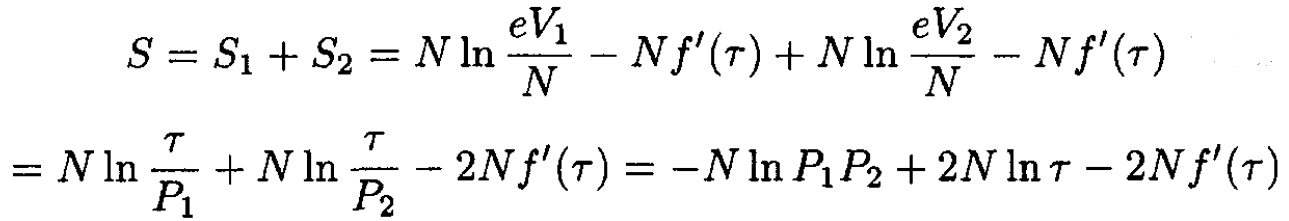 (8)
(8)
c) After the vessels are connected their volume becomes V = V1 + V2, the number of particles becomes 2N, and the temperature remains the same (no work is done in mixing the two gases). So now
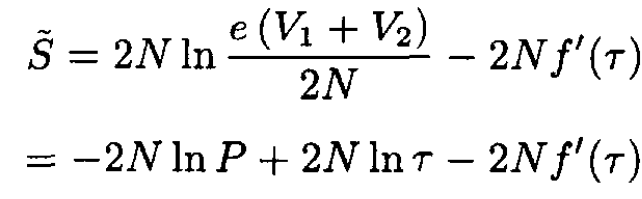 (9)
(9)
It can be easily seen that the pressure becomes 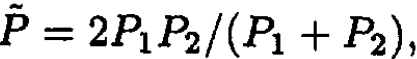 so
so
 (10)
(10)
and
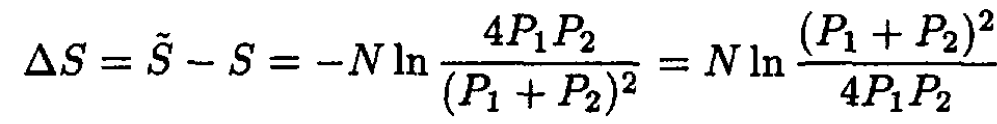 (11)
(11)
Let us show that ∆S is always nonnegative. This is equivalent to the condition
 (12)
(12)
which is always true. At P1 = P2 (V1 = V2), ∆S = 0, which makes perfect sense.
 الاكثر قراءة في مواضيع اخرى
الاكثر قراءة في مواضيع اخرى
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















