


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 17-12-2019
Date: 26-7-2016
Date: 17-12-2019
|
Enzymes Promote Sequences of Chemical Reactions
All biological macromolecules are much less thermodynamically stable than their monomeric subunits, yet they are kinetically stable: their uncatalyzed breakdown occurs so slowly (over years rather than seconds) that, on a time scale that matters for the organism, these molecules are stable. Virtually every chemical reaction in a cell occurs at a significant rate only because of the presence of enzymes—biocatalysts that, like all other catalysts, greatly enhance the rate of specific chemical reactions without being consumed in the process. The path from reactant(s) to product(s) almost invariably involves an energy barrier, called the activation barrier (Fig. 1–1), that must be surmounted for any reaction to proceed. The breaking of existing bonds and formation of new ones generally requires, first, the distortion of the existing bonds, creating a transition state of higher free energy than either reactant or product. The highest point in the reaction coordinate diagram represents the transition state, and the difference in energy between the reactant in its ground state and in its transition state is the activation energy, ΔG‡.An enzyme catalyzes a reaction by providing a more comfortable fit for the transition state: a surface that complements the transition state in stereochemistry, polarity, and charge. The binding of enzyme to the transition state is exergonic, and the energy released by this binding reduces the activation energy for the reaction and greatly increases the reaction rate. A further contribution to catalysis occurs when two or more reactants bind to the enzyme’s surface close to each other and with stereospecific orientations that favor the reaction. This increases by orders of magnitude the probability of productive collisions between reactants.
Again with a few exceptions, each enzyme catalyzes a specific reaction, and each reaction in a cell is catalyzed by a different enzyme. Thousands of different enzymes are therefore required by each cell. The multiplicity of enzymes, their specificity (the ability to discriminate between reactants), and their susceptibility to regulation give cells the capacity to lower activation barriers selectively. This selectivity is crucial for the effective regulation of cellular processes. By allowing specific reactions to proceed at significant rates at particular times, enzymes determine how matter and energy are channeled into cellular activities. The thousands of enzyme-catalyzed chemical reactions in cells are functionally organized into many sequences of consecutive reactions, called pathways, in which the product of one reaction becomes the reactant in the next. Some pathways degrade organic nutrients into simple end products in order to extract chemical energy and convert it into a form useful to the cell; together these degradative, free-energy-yielding reactions are designated catabolism. Other pathways start with small precursor molecules and convert them to progressively larger and more complex molecules, including proteins and nucleic acids. Such synthetic pathways, which invariably require the input of energy, are collectively designated anabolism.
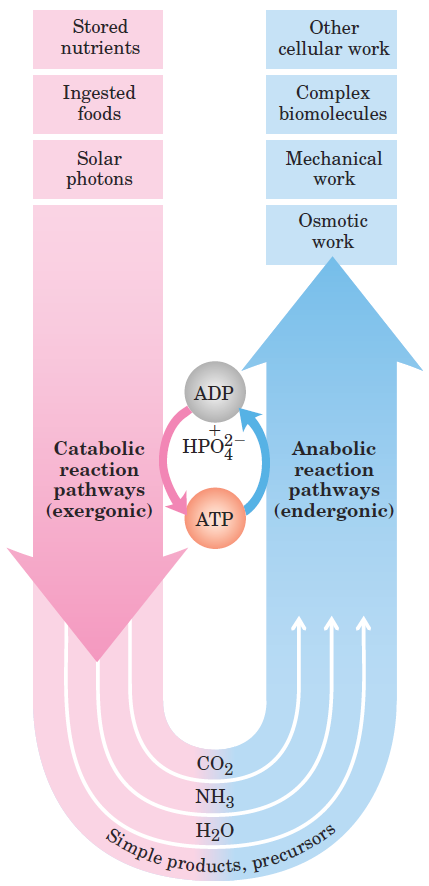
FIGURE 1–1 Energy changes during a chemical reaction. An activation barrier, representing the transition state, must be overcome in the conversion of reactants (A) into products (B), even though the products are more stable than the reactants, as indicated by a large, negative free-energy change (ΔG). The energy required to overcome the activation barrier is the activation energy (ΔG‡). Enzymes catalyze reactions by lowering the activation barrier. They bind the transitionstate intermediates tightly, and the binding energy of this interaction effectively reduces the activation energy from ΔG‡ uncat to ΔG‡ cat. (Note that activation energy is not related to free-energy change, ΔG.).
The overall network of enzyme-catalyzed pathways constitutes cellular metabolism. ATP is the major connecting link (the shared intermediate) between the catabolic and anabolic components of this network (shown schematically in Fig. 1–2). The pathways of enzyme-catalyzed reactions that act on the main constituents of cells—proteins, fats, sugars, and nucleic acids—are virtually identical in all living organisms.

FIGURE 1–2 The central role of ATP in metabolism. ATP is the shared chemical intermediate linking energy-releasing to energyrequiring cell processes. Its role in the cell is analogous to that of money in an economy: it is “earned/produced” in exergonic reactions and “spent/consumed” in endergonic ones.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|