


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 8-4-2021
Date: 16-12-2015
Date: 24-4-2021
|
Desmosomes, Desmocollin, Desmoglein, and Desmoplakin
Desmosomes are punctate, adhesive intercellular cell junctions that bind cells together and provide membrane anchoring points for the intermediate-filament cytoskeleton (Fig. 1). They are circular membrane domains of up to 0.5 µm in diameter. The intercellular material, or desmoglea, has a highly organized ultrastructure consisting of an electron-dense midline that is bridged to the plasma membranes of the adhering cells across a distance of ~30 nm. Close to the cytoplasmic faces of the plasma membranes are dense outer plaques, the most consistent and easily recognizable feature of these junctions, approximately 20 nm in thickness. At about 50 nm from the plasma membranes are less dense inner plaques that appear to associate with the intermediate filaments. Thus the total thickness of a desmosome from one inner plaque to the other is approximately 130 nm. The desmosomes may be thought of as the “scaffold couplings” that link the intermediate filament cytoskeleton throughout a tissue. Widely distributed, desmosomes are especially abundant in stratified epithelia, where strong intercellular adhesion is required to resist external friction. They are present in almost all epithelia, but also in cardiac muscle, the arachnoid and pia of the meninges, and the follicular dendritic cells of the lymphoid system.
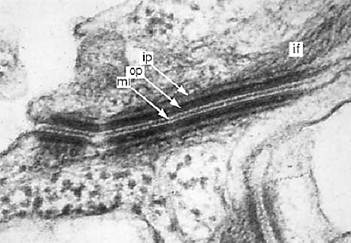
Figure 1. An electron micrograph of a desmosome from mouse tongue epithelium, showing the midline (ml), the outer and inner plaques (op and ip), and the intermediate filament (if) or tonofilament.
Desmosomes are macromolecular complexes consisting of at least six interacting proteins (Fig. 2). Their adhesion molecules are the desmocollins and desmogleins, members of the cadherin family of calcium-dependent cell adhesion molecules (1, 2). These transmembrane proteins have their extracellular domains in the desmoglea and their cytoplasmic domains in the dense outer plaques. The outer plaque contains two proteins that are members of the armadillo family named after the Drosophila segment polarity signaling gene. These are plakoglobin (3), also known as g-catenin and present in other intercellular junctions, and plakophilin (4). The outer and inner plaques are bridged by the plakin family member desmoplakin (5), which mediates interaction between the adhesive domain and the intermediate filaments. The most recently discovered desmosomal components are the two plakin family members, envoplakin (6) and periplakin (7), which although not exclusively epidermal, appear to be involved in cornified envelope formation.
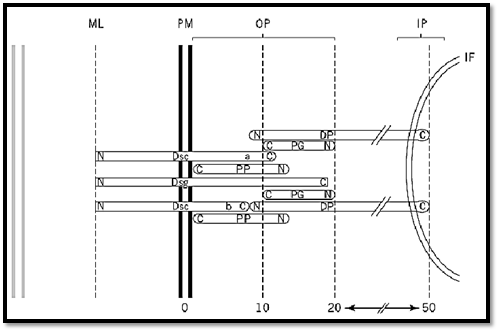
Figure 2. Diagram showing the major principle molecular components of the desmosome and their location within its structure. The diagram shows one-half of a demosome from the midline (ml) in the intercellular space, through the plasma membrane (pm) and the outer plaque (op), to the inner plaque (ip) and the intermediate filaments. The numbers at the bottom represent distances in nanometers from the plasma membrane. The amino-terminus (n) and the carboxy-terminus (c) of each component is indicated. The positions of these have been mapped by immuno-gold labeling with domain-specific antibodies and electron microscopy (unpublished observations). The components are: DP, desmoplakin; Dsc a, desmocollin “a” form; Dsc b, desmocollin “b” form; Dsg, desmoglein; PG, plakoglobin; PP, plakophilin.
1. Desmosomal Glycoproteins
There is direct evidence that the desmocollins (Dsc) and desmogleins (Dsg) mediate desmosomal adhesion, probably by a heterophilic interaction (8). Adhesive interaction probably involves tripeptide sequences known as “cell adhesion recognition sites” near the amino termini of the glycoproteins (9). These molecules represent two subfamilies of the cadherin superfamily, each occurring as three different isoforms and the products of different genes. The molecules resemble classical cadherins in their extracellular domains, but show unique features cytoplasmically. Desmocollins have alternatively spliced cytoplasmic domains [the longer “a” form and a shorter “b” form (10)], and the desmogleins have long cytoplasmic domains containing unique 29-amino-acid-residue repeats (11). The extracellular domains of these glycoproteins form an ordered array, giving rise to the highly structured desmosomal midline region or desmoglea. Their cytoplasmic domains contribute to the structure of the cytoplasmic plaque. The precise roles of the different cytoplasmic domains are unclear. That of the desmocollin “a” form can support plaque assembly and intermediate filament attachment (12), but the functions of desmoglein and desmocollin “b” in plaque assembly have not been resolved. Both desmocollin and desmoglein have been shown to bind plakoglobin and plakophilin, and desmocollin “a” can also bind desmoplakin. Three isoforms of desmocollin and desmoglein show tissue-specific expression (13, 14). Dsc2 and Dsg2 are ubiquitous in all desmosome-bearing tissues, but Dsc1, Dsg1, Dsc3, and Dsg3 are largely restricted to stratified epithelia. Desmosomal glycoprotein genes are closely linked on chromosome 18q12, and this is of possible significance for regulation of the expression.
2. Plakoglobin
Plakoglobin is a member of the armadillo family of proteins. It is not exclusively desmosomal but also occurs in adherens or intermediate junctions, even in non-desmosome-bearing tissues such as endothelia. It is, like b-catenin and armadillo itself, both a junctional protein and a member of the wingless Wnt signaling pathway (15). In its junctional role, it combines both with desmocollin and desmoglein, or with E-cadherin, where it is mutually exclusive with that of b-catenin. Null mutations of desmoplakin in mice result in severe disruption of desmosomes in cardiac muscle and epidermis. In its signaling role, it has been shown to produce axis duplication when overexpressed in Xenopus embryos and combined with the adenomatous polyposis coli (APC) protein that regulates its cytoplasmic level. The cytoplasmic plakoglobin can bind to the LEF-1 transcription factor to enter the nucleus and regulate gene activity (16).
3. Plakophilin
This is an armadillo family protein that has important junctional properties and possibly a signaling function. It exists as two isoforms that show differential tissue specific expression, PP1 being predominantly expressed in stratified tissues (17) and PP2 ubiquitously (18). The first human genetic disease resulting from a desmosomal mutation was an epidermal dysplasia/skin fragility syndrome, effectively a PP1 null mutation (19). This defect resulted in loss of adhesion between epidermal keratinocytes and detachment of intermediate filaments from the cell periphery, indicating an important role for plakophilin in linking between the desmosomal adhesion molecules and the cytoskeleton. Plakophilin has been shown to bind to both desmosomal glycoproteins, desmoplakin and cytokeratin (20). Its potential signaling role is inferred from its armadillo-type structure and because it has been detected in cell nuclei.
4. Desmoplakin
This is a plakin family member, together with plectin, bullous pemphigoid antigen 1 (BAPG1), envoplakin, and periplakin (6, 7). Except for BAPG1 all have been reported to be associated with desmosomes. Desmoplakin (DP) is a ubiquitous desmosomal component. DP1 is a coiled-coil dimer with globular end domains. The isolated molecule has an overall length of 130 nm. DP2 lacks the central coiled rod domain and is unable to dimerize. Its length is 43 nm. Expression studies, supported by immunogold labeling and electron microscopy
show that the amino-terminal globular domain associates with the desmosomal plaque and the carboxy-terminal domain with the intermediate filaments (21, 22). Thus desmoplakin plays an important role in linking between the desmosomal plaque and the cytoskeleton. Although not restricted to epidermis, envoplakin and periplakin were only identified as components of the cornified envelopes of suprabasal keratinocytes (6, 7). They are essentially similar in structure to desmoplakin, and their precise role has yet to be defined. The role of plectin is more problematic, because null mutations have no apparent effect on desmosome structure and function.
Desmosomal glycoproteins are target antigens in the autoimmune blistering disease of pemphigus (23) . Desmosomes appear at the 32- to 64-cell stage of embryonic development, where they are trophectoderm specific (24).
References
1. J. L. Holton et al. (1990) J. Cell Sci. 97, 239–246.
2. P. J. Koch et al. (1990) Eur. J. Cell Biol. 53, 1–12.
3. P. Cowin et al. (1986) Cell 1063–1073.
4. M. Hatzfeld et al. (1994) J. Cell Sci. 107, 2259–2270.
5. H. Mueller and W. W. Franke (1983) J. Mol. Biol. 163, 647–671.
6. C. Ruhrberg et al. (1996) J. Cell Biol. 134, 715–729.
7. C. Ruhrberg et al. (1997) J. Cell Biol. 139, 1835–1849.
8. N. A. Chitaev and S. M. Troyanovsky (1997) J. Cell Biol. 138, 193–201.
9. C. Tselepis et al. (1998) Proc. Natl. Acad. Sci. USA 95, 8064–8069.
10. J. E. Collins et al. (1991) J. Cell Biol. 113, 381–391.
11. G. N. Wheeler et al. (1991) Proc. Natl. Acad. Sci. USA 88, 4796–4800.
12. S. Troyanovsky et al. (1993) Cell 72, 561–574.
13. P. K. Legan et al. (1994) J. Cell Biol. 126, 507–518.
14. S. Schäfer, P. J. Koch, and W. W. Franke (1994) Exp. Cell Res. 211, 391–399.
15. P. Cowin and B. Burke (1996) Curr. Opin. Cell Biol. 8, 56–65.
16. O. Huber et al. (1996) Mech. Dev. 59, 3–10.
17. H. W. Heid et al. (1994) Differentiation 58, 113–131.
18. C. Mertens et al. (1996) J. Cell Biol. 135, 1009–1025.
19. J. McGrath et al. (1997) Nature Genet. 17, 240–244.
20. E. A. Smith and E. Fuchs (1998) J. Cell Biol. 141, 1229–1241.
21. E. A. Bornslaeger et al. (1996) J. Cell Biol. 134, 985–1001.
22. A. P. Kowalczyk et al. (1997) J. Cell Biol. 139, 773–784.
23. J. R. Stanley (1995) In “Cell adhesion and human disease.” Ciba Found. Symp. 189, 107–120.
24. T. P. Fleming et al. (1991) Development 112, 527–529.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ندوات وأنشطة قرآنية مختلفة يقيمها المجمَع العلمي في محافظتي النجف وكربلاء
|
|
|