The Nucleophilic Reagent
The nucleophilicty of a particular reagent (:Y, :Y⊖ , or HY¨) can be defined as its ability to donate an electron pair to another atom . In fact, the SN2 reactivity of a reagent toward a methyl derivative can be taken to measure its nucleophilicty toward carbon. The relative reaction rates of some nucleophiles toward methyl bromide are listed in order of increasing nucleophilicity in Table 8-4, together with their basicities as measured by Kb. Important generalizations can be made from these data provided that one recognizes that they may apply only to hydroxylic solvents.
1. For the atoms representing any one group (column) of the periodic table, nucleophilicty increases with increasing atomic number: I⊖ > Br⊖ > Cl⊖ > F⊖; HS⊖ > HO⊖; PH3 > NH3. Thus, other things being equal, larger atoms are better nucleophiles
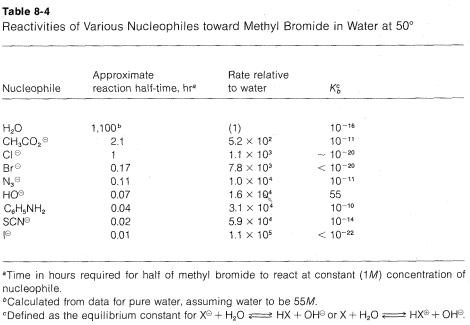
2. For nucleophiles having the same atomic number of the entering atom (e.g., oxygen nucleophiles), there is usually a good correlation between the basicity of the reagent and its nucleophilicity. Thus a weak base such as CH3CO2⊖ is a poorer nucleophile than a strong base such as ⊖OH. The poorer X⊖ is as a leaving group, the better it is as an entering group.
3. For nucleophiles of different atomic numbers, nucleophilicity usually does not parallel basicity. For example, for the halogens the reactivity sequence I⊖ > Br⊖ > Cl⊖ is opposite to the sequence for basicity Cl⊖ > Br⊖ > I⊖. Similarly, sulfur anions such as HS⊖ are better nucleophiles but weaker bases than corresponding oxyanions such as HO⊖.
4. A number of nucleophilic agents, which are very reactive in SN2 reactions, are of the type X−Y, where both atoms have unshared electron pairs. Examples include HOO⊖, H2NO⊖, ClO⊖, and H2NNH2, all of which are more reactive than the closely related nucleophiles HO⊖ and NH3.
Why is the correlation between basicity and nucleophilcity so poor for atoms of different atomic number? it is now clear from much research that the dominant effect is associated with differences in the solvation energies of the ions, as defined for halide ions by the following equations:
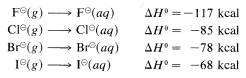
The solvation energies of small ions with concentrated charge always are greater than those of large ions with diffuse charge.
When an ion participates in a nucleophilic attack on carbon, it must slough off some of the solvent molecules that stabilize it in solution. Otherwise, the ion cannot get close enough to the carbon, to which it will become attached, to begin forming a bond. Sloughing off solvent molecules will be less favorable for a small ion than a large ion. Consequently, we expect Cl⊖ to be less reactive than I⊖.
Strong evidence for solvation effects on reactivity is provided by the fact that chloride ion is more reactive than iodide in solvents that have low solvation energies for anions. Furthermore, in the gas phase where solvation effects are absent, F⊖ is more reactive than any of the other halide ions toward chloromethane:
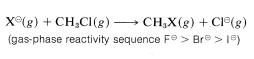
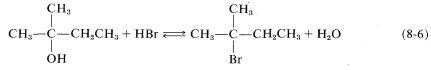
It should be recognized that SN reactions may be reversible when both the leaving group X and the entering group Y are good entering and leaving groups, respectively. In such circumstances, the position of the equilibrium often can be changed by suitably adjusting the reaction conditions. Thus 48% aqueous hydrogen bromide can convert alcohols to alkyl bromides (Equation 8-6, forward direction), whereas the reverse reaction (hydrolysis) is achieved by high water concentration: