


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 15-10-2020
التاريخ: 27-8-2019
التاريخ: 11-9-2020
التاريخ: 10-9-2020
|
The length of a chemical bond is the average distance between the nuclei of two bonded atoms, regardless of where the bonding electrons happen to be. The customary unit of length is the angstrom11 (A=10−10 m), and measurements often can be made with an accuracy of 0.001A by using the techniques of molecular spectroscopy, x-ray diffraction (for crystalline solids), and electron diffraction (for volatile compounds). Bond lengths vary considerably with structure and depend on the identity of both atoms, the type of bonding (single, double, or triple), and the nature of other atoms or groups bonded to the two atoms in question. These effects are apparent in the data of Table 2-1, which lists the bond lengths in several simple organic compounds. Multiple bonds, double or triple, clearly are shorter than single bonds, and it can be stated as a general observation that the more bonding electrons in a given bond, the shorter (and stronger) the bond. The lengths of single C−C bonds also vary significantly depending on what other atoms or groups are attached to the carbons. Thus Table 2-1 shows that single C−C bonds become progressively shorter as the number of multiple bonds or electronegative atoms attached to the carbons increases.
Although molecular models cannot represent the subtle variations in bond lengths and bond angles that actual molecules exhibit, most kinds of commercially available molecular models do attempt to reproduce relative bond lengths with some degree of reality. In the ball-and-stick type, the sticks usually come in various lengths to simulate different kinds of bonds; C−H bonds typically are scaled to represent 1.1A, C−C bonds to be 1.54A, and C=C and C≡C to be correspondingly shorter. In some model sets the bonds can be cut to any desired length.
While the ball-and-stick models of molecules are very useful for visualizing the relative positions of the atoms in space, they are unsatisfactory whenever we also want to show how large the atoms are. Actually, atomic radii are so large relative to the lengths of chemical bonds that when a model of a molecule such as methyl chloride is constructed with atomic radii and bond lengths, both to scale, the bonds connecting the atoms are not clearly evident. None-
Figure 2-3 shows how the CPK models can indicate intense molecular crowding, as between the bromines in 1,8-dibromonaphthalene:
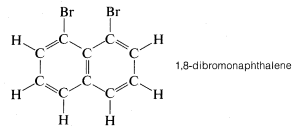
As we shall see, such crowding has many chemical consequences.
Ideally, a model should reflect not only the size and shape of the molecule it represents but also the flexibility of the molecule. By this we mean that
Figure 2-3: CPK space-filling models of organic compounds
it should simulate the type of motions available to the molecule, particularly bond rotation. For example, it is known that rotation normally occurs about single bonds in open-chain compounds but is restricted about double bonds. Motions of this kind are demonstrated easily with ball-and-stick models, but are not at all obvious with the space-filling type. For this reason, ball-and-stick models or their equivalent are more generally useful than the space-filling models for visualizing structures and the positions of the atoms relative to one another.
The angstrom unit likely will be replaced eventually by the nanometer (1nm=10−9 m=1 nm=10−9 =10 A).



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|