


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 19-12-2021
التاريخ: 16-12-2021
التاريخ: 16-12-2021
التاريخ: 18-12-2021
|
The first antibiotics of medicinal value were discovered by Alexander Fleming in 1929 as metabolites of the microorganism Penicillium notatum. They became known as penicillins, but their development as useful drugs was slow in coming. However, the urgent need for nontoxic antibiotics was recognized during World War II, and resulted in a team effort by English and American scientists to develop efficient methods for preparing penicillin by fermentation and to undertake clinical and chemical studies. By 1943, penicillin was available in quantity for the treatment of war wounded. By 1945, the basic structure and stereochemistry was deduced through chemical degradation and x-ray diffraction studies:
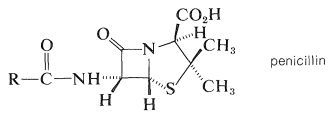
The structure is unusual in that it has a four-membered cyclic amide ring (β-lactam). It was the first example to be discovered of a natural product with this ring structure.
Fermentation can produce penicillins that differ only in the nature of the side-chain group RR. The common natural penicillin is penicillin G, in which R=R= phenylmethyl (benzyl):
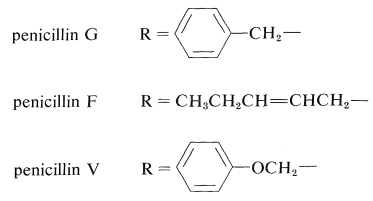
The cephalosporins are antibiotics produced by the bacterial strain cephalosporium. They are closely related to the penicillins. Thus cephalosporin C has a β-lactam ring but a six-membered sulfur-containing ring:
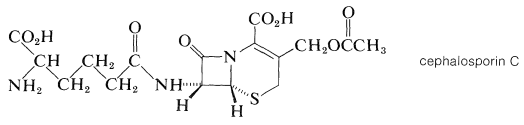
Both the cephalosporins and the penicillins owe their antibacterial action to their ability to block bacterial cell-wall biosynthesis. Cephalosporin C is less active than the penicillins, but is less susceptible to enzymatic destruction by β-lactamases, which are enzymes that cleave the lactam ring. In fact, the so-called resistance of staph bacteria to penicillins is attributed to the propagation of strains that produce β-lactamase. Numerous semisynthetic penicillins and cephalosporins have been made in the hope of finding new broad-spectrum antibiotics with high activity but with greater β-lactam stability. Several of these are in clinical use.
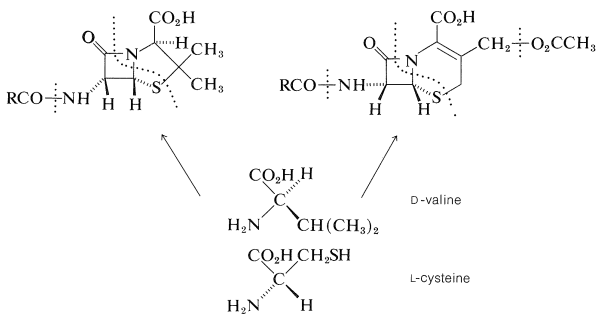
The total synthesis of penicillin V was achieved by J. C. Sheehan (1957) and of cephalosporin by R. B. Woodward (1966). Biosynthetic routes have been worked out in part, and the precursors to both ring systems are L-cysteine and D-valine:



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
بالصور: تزامنا مع ختام فعالياته.. ممثل المرجعية العليا يشارك في المحفل القرآني المركزي في الصحن الحسيني الشريف
|
|
|