


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 16-9-2019
Date: 27-9-2020
Date: 23-1-2020
|
Since the negative charge of an enolate anion is delocalized over the alpha-carbon and the oxygen, electrophiles may bond to either atom. Reactants having two or more reactive sites are called ambident, so this term is properly applied to enolate anions. Modestly electrophilic reactants such as alkyl halides are not sufficiently reactive to combine with neutral enol tautomers, but the increased nucleophilicity of the enolate anion conjugate base permits such reactions to take place. Because alkylations are usually irreversible, their products should reflect the inherent (kinetic) reactivity of the different nucleophilic sites.
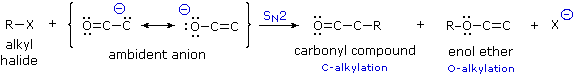
If an alkyl halide undergoes an SN2 reaction at the carbon atom of an enolate anion the product is an alkylated aldehyde or ketone. On the other hand, if the SN2 reaction occurs at oxygen the product is an ether derivative of the enol tautomer; such compounds are stable in the absence of acid and may be isolated and characterized. These alkylations (shown above) are irreversible under the conditions normally used for SN2 reactions, so the product composition should provide a measure of the relative rates of substitution at carbon versus oxygen. It has been found that this competition is sensitive to a number of factors, including negative charge density, solvation, cation coordination and product stability.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|