


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية | Substituents determine the reaction direction by resonance or inductive effect |
|
|
|
Read More
Date: 12-12-2020
Date: 28-10-2020
Date: 27-9-2019
|
Resonance effect is the conjugation between the ring and the substituent, which means the delocalizing of the π electrons between the ring and the substituent. Inductive effect is the withdraw of the sigma ( the single bond ) electrons away from the ring toward the substituent, due to the higher electronegativity of the substituent compared to the carbon of the ring.
When the substituents like -OH have an unshared pair of electrons, the resonance effect is stronger than the inductive effect which make these substituents stronger activators, since this resonance effect direct the electron toward the ring. In cases where the subtituents is esters or amides, they are less activating because they form resonance structure that pull the electron density away from the ring.
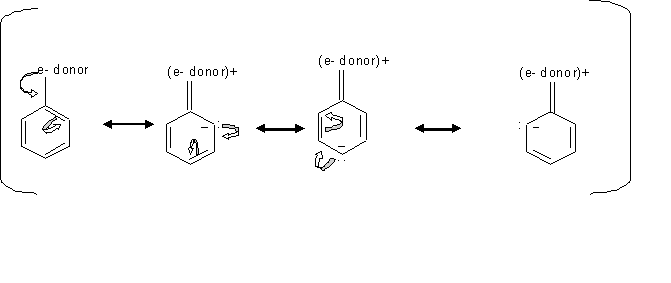
By looking at the mechanism above, we can see how groups donating electron direct the ortho, para electrophilic substition. Since the electrons locatinn transfer between the ortho and para carbons, then the electrophile prefer attacking the carbon that has the free electron.
Inductive effect of alkyl groups activates the direction of the ortho or para substitution, which is when s electrons gets pushed toward the ring.



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|