


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 29-10-2020
Date: 21-10-2020
Date: 7-9-2018
|
In biological reactions, we do not often see halides serving as leaving groups (in fact, outside of some marine organisms, halogens are fairly unusual in biological molecules). More common leaving groups in biochemical reactions are phosphates, water, alcohols, and thiols. In many cases, the leaving group is protonated by an acidic group on the enzyme as bond-breaking occurs. For example, hydroxide ion itself seldom acts as a leaving group – it is simply too high in energy (too basic). Rather, the hydroxide oxygen is generally protonated by an enzymatic acid before or during the bond-breaking event, resulting in a (very stable) water leaving group.
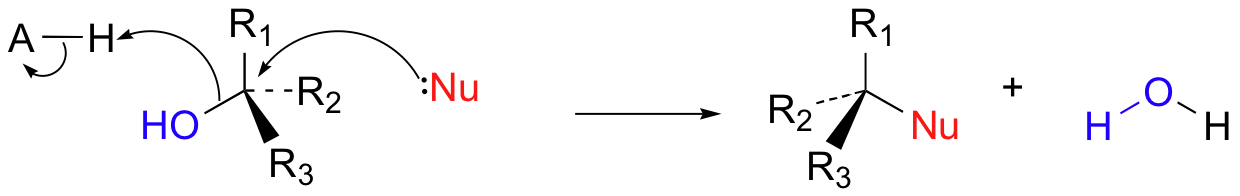
More often, however, the hydroxyl group of an alcohol is first converted enzymatically to a phosphate ester in order to create a better leaving group. This phosphate ester can take the form of a simple monophosphate (arrow 1 in the figure below), a diphosphate (arrow 2), or a nucleotide monophosphate (arrow 3).
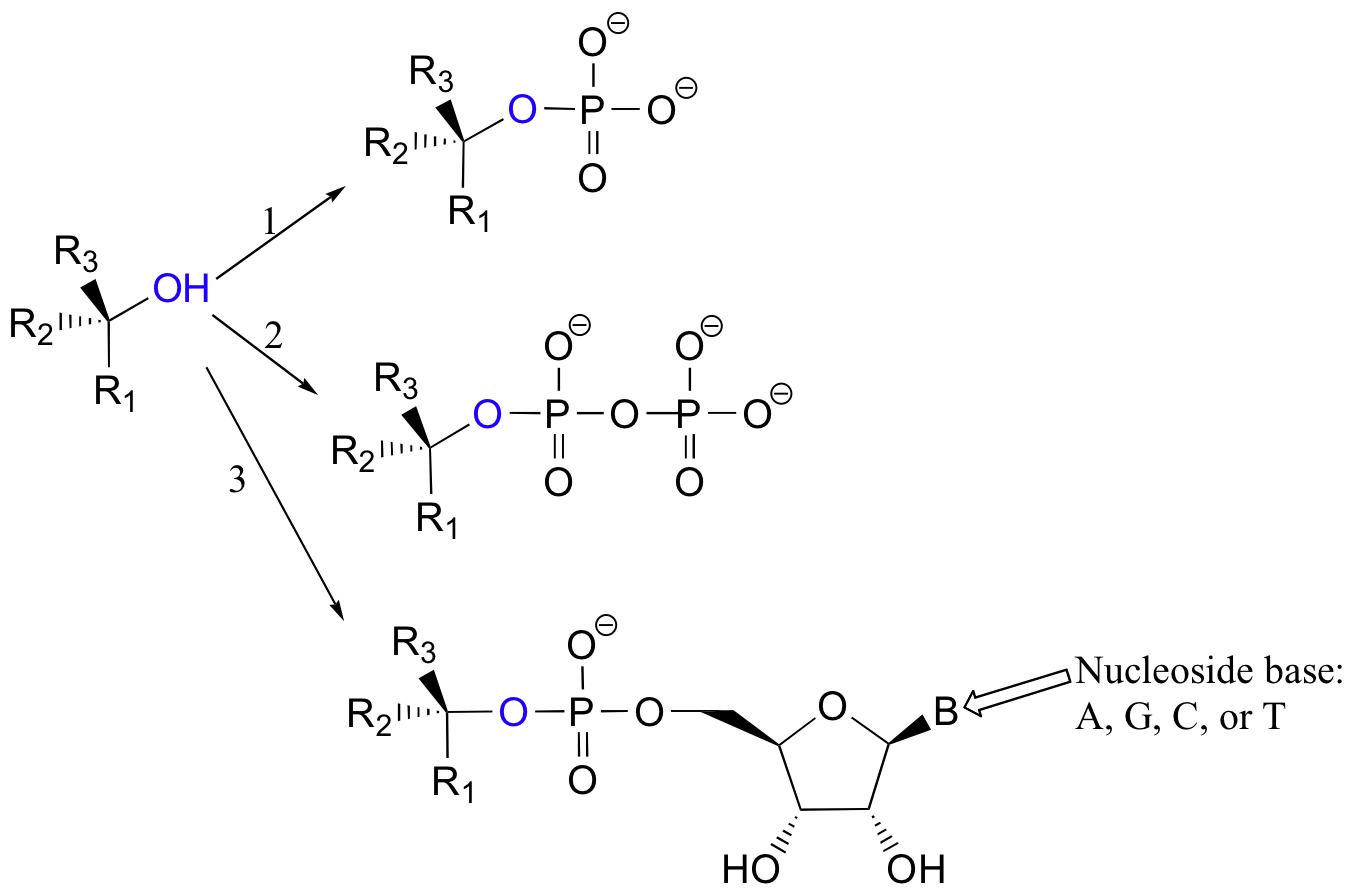
Due to resonance delocalization of the developing negative charge, phosphates are excellent leaving groups.

Here’s a specific example (from DNA nucleotide biosynthesis) that we will encounter in more detail in section 11.5:
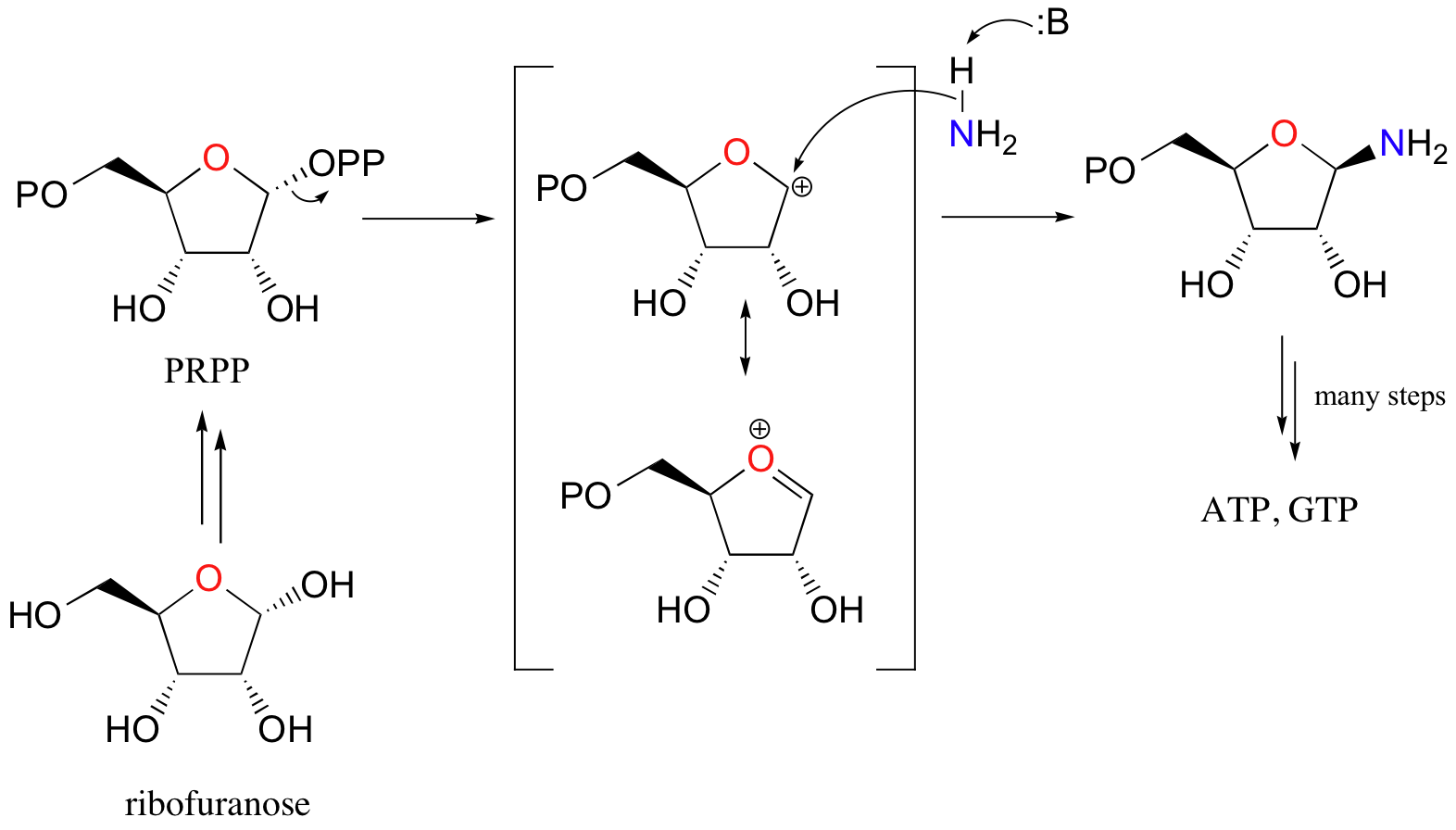
Here, the OH group on ribofuranose is converted to a diphosphate, a much better leaving group. Ammonia is the nucleophile in the second step of this SN1-like reaction.
What is important for now is that in each case, an alcohol has been converted into a much better leaving group, and is now primed for a nucleophilic substitution reaction.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|