


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-9-2020
Date: 8-9-2020
Date: 10-9-2020
|
Trigonal planar, sp2-hybridized carbons are not, as we well know, chiral centers– but they are referred to as prochiral centers if they are bonded to three different substitutuents. As you might expect, we (and the enzymes that catalyze their reactions) can distinguish between the two planar ‘faces’ of a prochiral sp2 - hybridized group. These faces are designated by the terms re and si. To determine which is the re and which is the si face of a planar organic group, we simply use the same priority rankings that we are familiar with from the R/S system, and trace a circle: re is clockwise and si is counterclockwise.
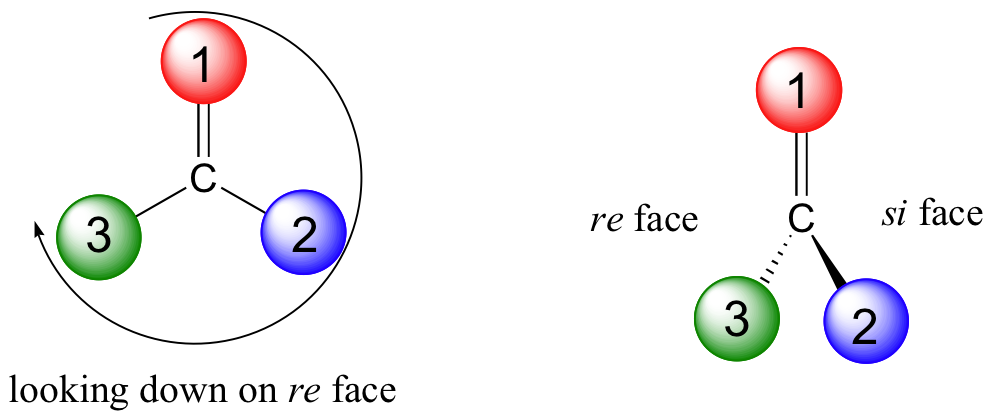
Below, for example, we are looking down on the re face of the ketone group in pyruvate:
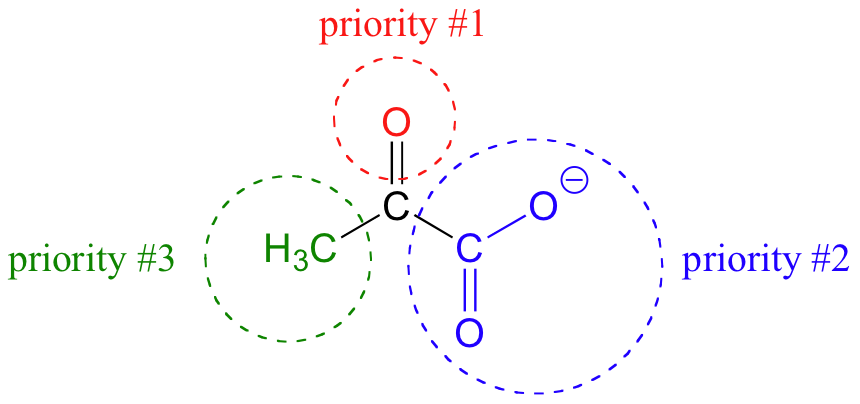
If we flipped the molecule over, we would be looking at the si face of the ketone group. Note that the carboxylate group does not have re and si faces, because two of the three substituents on that carbon are identical (when the two resonance forms of carboxylate are taken into account).
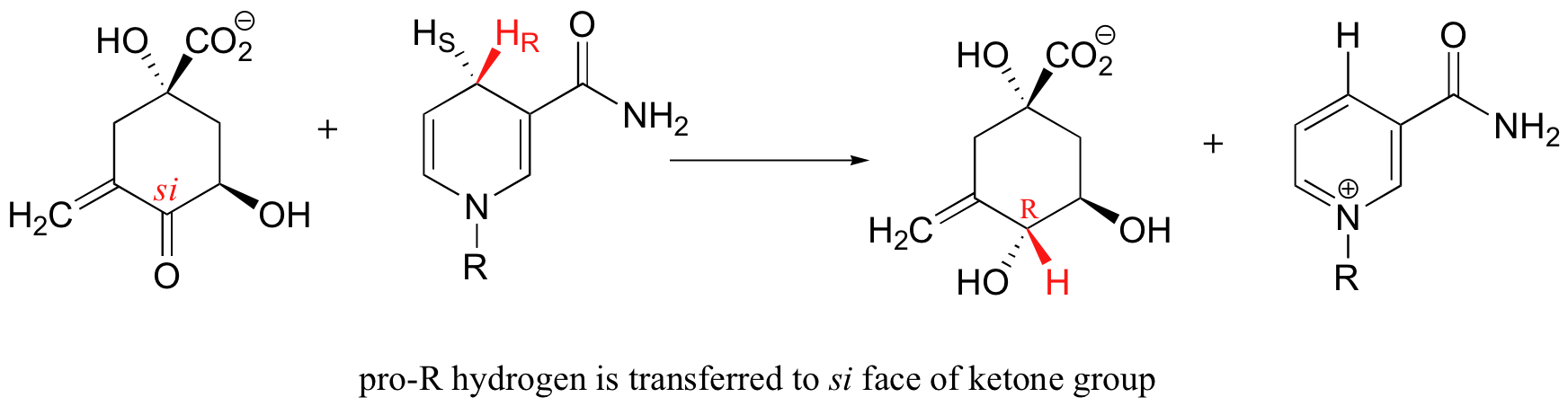
As we will see beginning in chapter 11, enzymes which catalyze reactions at carbonyl carbons act specifically from one side or the other.



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
لحماية التراث الوطني.. العتبة العباسية تعلن عن ترميم أكثر من 200 وثيقة خلال عام 2024
|
|
|