


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 21-5-2019
Date: 8-8-2016
Date: 8-8-2016
|
A measurement usually consists of a unit and a number expressing the quantity of that unit. We may express the same physical measurement with different units, which can create confusion. For example, the mass of a sample weighing 1.5 g also may be written as 0.0033 lb or 0.053 oz. To ensure consistency, and to avoid problems, scientists use a common set of fundamental units, several of which are listed in Table 1.1 . These units are called SI units after the Système International d’Unités.
It is important for scientists to agree upon a common set of units. In 1999 NASA lost a Mar’s Orbiter spacecraft because one engineering team used English units and another engineering team used metric units. As a result, the spacecraft came too close to the planet’s surface, causing its propulsion system to overheat and fail.
Some measurements, such as absorbance, do not have units. Because the meaning of a unitless number may be unclear, some authors include an artificial unit. It is not unusual to see the abbreviation AU, which is short for absorbance unit, following an absorbance value. Including the AU clarifies that the measurement is an absorbance value.
We define other measurements using these fundamental SI units. For example, we measure the quantity of heat produced during a chemical reaction in joules, (J), where
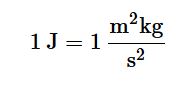
Table 1.2 provides a list of some important derived SI units, as well as a few common non-SI units.
| Measurement | Unit | Symbol | Definition (1 unit is...) |
|---|---|---|---|
| mass | kilogram | kg | ...the mass of the international prototype, a Pt-Ir object housed at the Bureau International de Poids and Measures at Sèvres, France.† |
| distance | meter | m | ...the distance light travels in (299 792 458)-1 seconds. |
| temperature | Kelvin | K | ...equal to (273.16)–1, where 273.16 K is the triple point of water (where its solid, liquid, and gaseous forms are in equilibrium). |
| time | second | s | ...the time it takes for 9 192 631 770 periods of radiation corresponding to a specific transition of the 133Cs atom. |
| current | ampere | A | ...the current producing a force of 2 × 10-7 N/m when maintained in two straight parallel conductors of infinite length separated by one meter (in a vacuum). |
| amount of substance | mole | mol | ...the amount of a substance containing as many particles as there are atoms in exactly 0.012 kilogram of 12C. |
† The mass of the international prototype changes at a rate of approximately 1 mg per year due to reversible surface contamination. The reference mass, therefore, is determined immediately after its cleaning by a specified procedure.
| Measurement | Unit | Symbol | Equivalent SI Units |
|---|---|---|---|
| length | angstrom (non-SI) | Å | 1 Å = 1 × 10–10 m |
| volume | liter (non-SI) | L | 1 L = 10–3 m3 |
| force | newton (SI) | N | 1 N = 1 m·kg/s2 |
| pressure | pascal (SI) | Pa | 1 Pa = 1 N/m2 = 1 kg/(m·s2) |
| atmosphere (non-SI) | atm | 1 atm = 101,325 Pa | |
| energy, work, heat | joule (SI) | J | 1 J = N·m = 1 m2·kg/s2 |
| calorie (non-SI) | cal | 1 cal = 4.184 J | |
| electron volt (non-SI) | eV | 1 eV = 1.602 177 33 × 10–19 J | |
| power | watt (SI) | W | 1 W = 1 J/s = 1 m2·kg/s3 |
| charge | coulomb (SI) | C | 1 C = 1 A·s |
| potential | volt (SI) | V | 1 V = 1 W/A = 1 m2·kg/(s3·A) |
| frequency | hertz (SI) | Hz | 1 Hz = s–1 |
| temperature | Celsius (non-SI) | oC | oC = K – 273.15 |
Chemists frequently work with measurements that are very large or very small. A mole contains 602 213 670 000 000 000 000 000 particles and some analytical techniques can detect as little as 0.000 000 000 000 001 g of a compound. For simplicity, we express these measurements using scientific notation; thus, a mole contains 6.022 136 7 × 1023 particles, and the detected mass is 1 × 10–15 g. Sometimes it is preferable to express measurements without the exponential term, replacing it with a prefix (Table 2.3). A mass of 1×10–15 g, for example, is the same as 1 fg, or femtogram.
Writing a lengthy number with spaces instead of commas may strike you as unusual. For numbers containing more than four digits on either side of the decimal point, however, the currently accepted practice is to use a thin space instead of a comma.
| Prefix | Symbol | Factor | Prefix | Symbol | Factor | Prefix | Symbol | Factor |
|---|---|---|---|---|---|---|---|---|
| yotta | Y | 1024 | kilo | k | 103 | micro | μ | 10–6 |
| zetta | Z | 1021 | hecto | h | 102 | nano | n | 10–9 |
| eta | E | 1018 | deka | da | 101 | pico | p | 10-12 |
| peta | P | 1015 | - | - | 100 | femto | f | 10–15 |
| tera | T | 1012 | deci | d | 10–1 | atto | a | 10–18 |
| giga | G | 109 | centi | c | 10–2 | zepto | z | 10–21 |
| mega | M | 106 | milli | m | 10–3 | yocto | y | 10–24 |



|
|
|
|
5 علامات تحذيرية قد تدل على "مشكل خطير" في الكبد
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|