


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 15-1-2020
Date: 31-10-2019
Date: 10-7-2018
|
Epoxides (oxiranes) are three-membered cyclic ethers that are easily prepared from alkenes by reaction with peracids. Because of the large angle strain in this small ring, epoxides undergo acid and base-catalyzed C–O bond cleavage more easily than do larger ring ethers. Among the following examples, the first is unexceptional except for the fact that it occurs under milder conditions and more rapidly than other ether cleavages. The second and third examples clearly show the exceptional reactivity of epoxides, since unstrained ethers present in the same reactant or as solvent do not react. The aqueous acid used to work up the third reaction, following the Grignard reagent cleavage of the ethylene oxide, simply neutralizes the magnesium salt of the alcohol product.
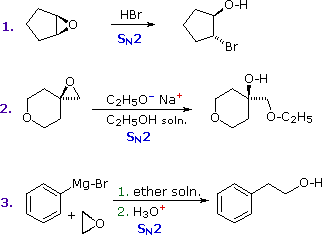



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|