


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 31-5-2016
Date: 15-12-2016
Date: 1-10-2019
|
Preparation and Reactions of Silyl Enol Ethers
One way of producing selective enolate anion intermediates is to first trap and isolate them as silyl enol ethers. These relatively stable compounds may then be used to generate isomerically pure enolate anions, or in some cases as enolic nucleophiles in their own right. In the following diagram, the first reaction illustrates the formation of a mixture of silyl enol ethers under equilibrating conditions. If a higher proportion of the minor isomer is desired the kinetically favored lithium enolate can be prepared and quenched with trimethylsilyl chloride. In either case the silyl ether mixture may be separated by distillation. Once a pure silyl ether isomer is in hand, it may be used to generate the corresponding lithium enolate in the manner shown. Alkylation reactions of these enolates then produces pure regioisomeric products.
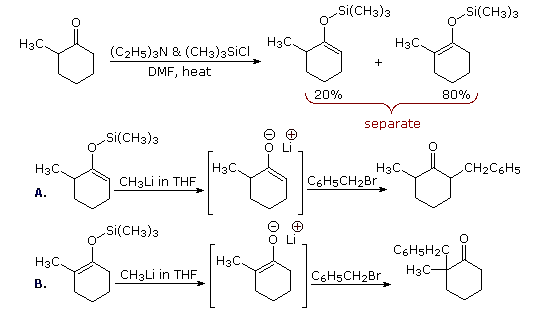
By clicking the "Toggle Reactions" button under the previous diagram, two examples of the direct use of silyl enol ethers will be displayed. Since the silyl ethers are not as reactive as enolate anions, the electrophiles with which they combine must be made more reactive. When carbonyl electrophiles are used, this can be accomplished by Lewis acid catalysts, as shown.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|