

النبات

مواضيع عامة في علم النبات

الجذور - السيقان - الأوراق

النباتات الوعائية واللاوعائية

البذور (مغطاة البذور - عاريات البذور)

الطحالب

النباتات الطبية


الحيوان

مواضيع عامة في علم الحيوان

علم التشريح

التنوع الإحيائي

البايلوجيا الخلوية


الأحياء المجهرية

البكتيريا

الفطريات

الطفيليات

الفايروسات


علم الأمراض

الاورام

الامراض الوراثية

الامراض المناعية

الامراض المدارية

اضطرابات الدورة الدموية

مواضيع عامة في علم الامراض

الحشرات


التقانة الإحيائية

مواضيع عامة في التقانة الإحيائية


التقنية الحيوية المكروبية

التقنية الحيوية والميكروبات

الفعاليات الحيوية

وراثة الاحياء المجهرية

تصنيف الاحياء المجهرية

الاحياء المجهرية في الطبيعة

أيض الاجهاد

التقنية الحيوية والبيئة

التقنية الحيوية والطب

التقنية الحيوية والزراعة

التقنية الحيوية والصناعة

التقنية الحيوية والطاقة

البحار والطحالب الصغيرة

عزل البروتين

هندسة الجينات


التقنية الحياتية النانوية

مفاهيم التقنية الحيوية النانوية

التراكيب النانوية والمجاهر المستخدمة في رؤيتها

تصنيع وتخليق المواد النانوية

تطبيقات التقنية النانوية والحيوية النانوية

الرقائق والمتحسسات الحيوية

المصفوفات المجهرية وحاسوب الدنا

اللقاحات

البيئة والتلوث


علم الأجنة

اعضاء التكاثر وتشكل الاعراس

الاخصاب

التشطر

العصيبة وتشكل الجسيدات

تشكل اللواحق الجنينية

تكون المعيدة وظهور الطبقات الجنينية

مقدمة لعلم الاجنة


الأحياء الجزيئي

مواضيع عامة في الاحياء الجزيئي


علم وظائف الأعضاء


الغدد

مواضيع عامة في الغدد

الغدد الصم و هرموناتها

الجسم تحت السريري

الغدة النخامية

الغدة الكظرية

الغدة التناسلية

الغدة الدرقية والجار الدرقية

الغدة البنكرياسية

الغدة الصنوبرية

مواضيع عامة في علم وظائف الاعضاء

الخلية الحيوانية

الجهاز العصبي

أعضاء الحس

الجهاز العضلي

السوائل الجسمية

الجهاز الدوري والليمف

الجهاز التنفسي

الجهاز الهضمي

الجهاز البولي


المضادات الميكروبية

مواضيع عامة في المضادات الميكروبية

مضادات البكتيريا

مضادات الفطريات

مضادات الطفيليات

مضادات الفايروسات

علم الخلية

الوراثة

الأحياء العامة

المناعة

التحليلات المرضية

الكيمياء الحيوية

مواضيع متنوعة أخرى

الانزيمات
Most Eukaryotic mRNA Is Degraded via Two Deadenylation-Dependent Pathways
المؤلف:
JOCELYN E. KREBS, ELLIOTT S. GOLDSTEIN and STEPHEN T. KILPATRICK
المصدر:
LEWIN’S GENES XII
الجزء والصفحة:
18-5-2021
1927
Most Eukaryotic mRNA Is Degraded via Two Deadenylation-Dependent Pathways
KEY CONCEPTS
- The modifications at both ends of mRNA protect it against degradation by exonucleases.
- The two major mRNA decay pathways are initiated by deadenylation catalyzed by poly(A) nucleases.
- Deadenylation may be followed either by decapping and 5′ to 3′ exonuclease digestion or by 3′ to 5′ exonuclease digestion.
- The decapping enzyme competes with the translation initiation complex for 5′ cap binding.
- The exosome, which catalyzes 3′ to 5′ mRNA digestion, is a large, evolutionarily conserved complex.
- Degradation may occur within discrete cytoplasmic particles called processing bodies (PBs).
- A variety of particles containing translationally repressed mRNAs exist in different cell types.
Eukaryotic mRNAs are protected from exonucleases by their modified ends . The 7-methyl guanosine cap protects against 5′ attack; the poly(A) tail, in association with bound proteins, protects against 3′ attack. Exceptions are the histone mRNAs in mammals, which terminate in a stem-loop structure rather than a poly(A) tail. A sequence-independent endonuclease attack—the initiating mechanism used by bacteria—is rare or absent in eukaryotes. mRNA decay has been characterized most extensively in budding yeast, although most findings apply to mammalian cells as well.
Degradation of the vast majority of mRNAs is deadenylation dependent; that is, degradation is initiated by breaching their protective poly(A) tail. The newly formed poly(A) tail (which is about 70 to 90 adenylate nucleotides in yeast and about 200 in mammals) is coated with poly(A)-binding proteins (PABPs). The poly(A) tail is subject to gradual shortening upon entry into the cytoplasm, a process catalyzed by specific poly(A) nucleases (also called deadenylases). In both yeast and mammalian cells, the poly(A) tail is initially shortened by the PAN2/3 complex, followed by a more rapid digestion of the remaining 60- to 80-A tail by a second complex, CCR4-NOT, which contains the processive exonuclease CCR4 and at least eight other subunits. Remarkably, similar CCR4-NOT complexes are involved in a variety of other processes in gene expression, including transcriptional activation. It is thought to be a global regulator of gene expression, integrating transcription and mRNA degradation. Other poly(A) nucleases exist in both yeast and mammalian cells, and the reason for this multiplicity is not yet clear.
Two different mRNA degradation pathways are initiated by poly(A) removal, as shown in FIGURE 1. In the first pathway (Figure 20.6, left), digestion of the poly(A) tail down to oligo(A) length (10 to 12 As) triggers decapping at the 5′ end of the mRNA. Decapping is catalyzed by a decapping enzyme complex consisting of two proteins in yeast (Dcp1 and Dcp2) and their homologs plus additional proteins in mammals. Decapping yields a 5′ monophosphorylated RNA end (the substrate for the 5′ to 3′ processive exonuclease Xrn1), which rapidly digests the mRNA. In fact, this digestion is so fast that intermediates could not be identified until investigators discovered that a stretch of guanosine nucleotides (poly[G]) could block Xrn1 progression in yeast. As illustrated in FIGURE 2, they engineered mRNAs to contain an internal poly(G) tract and found that the oligoadenylated 3′ end of the mRNAs accumulated. This result showed that 5′ to 3′ exonuclease digestion is the primary route of decay and that decapping precedes complete removal of the poly(A) tail.
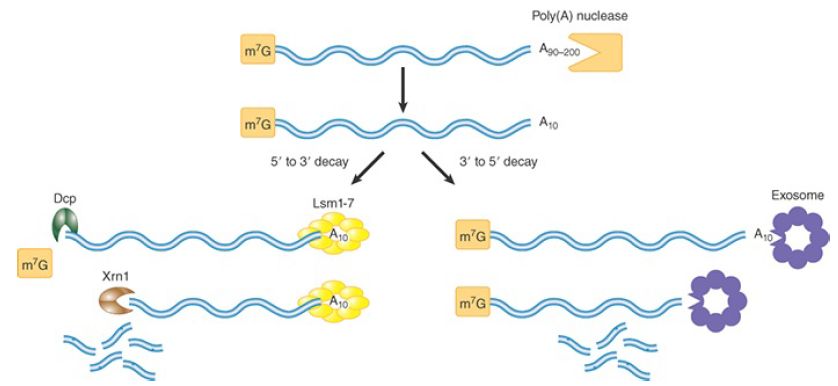
FIGURE 1.The major deadenylation-dependent decay pathways in eukaryotes. Two pathways are initiated by deadenylation. In both, poly(A) is shortened by a poly(A) nuclease until it reaches a length of about 10 A. Then an mRNA may be degraded by the 5′ to 3′ pathway or by the 3′ to 5′ pathway. The 5′ to 3′ pathway involves decapping by Dcp and digestion by the Xrn1 exonuclease. The 3′ to 5′ pathway involves digestion by the exosome complex.
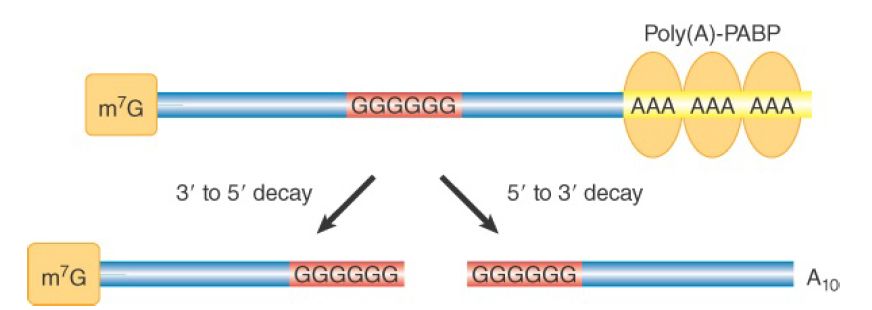
FIGURE 20.7 Use of a poly(G) sequence to determine direction of decay. A poly(G) sequence, engineered into an mRNA, will block the progression of exonucleases in yeast. The 5′ or 3′ mRNA fragment resistant to degradation accumulates in the cell and can be identified by northern blot.
The cap is normally resistant to decapping during active translation because it is bound by the cytoplasmic cap-binding protein, a component of the eukaryotic initiation factor 4F (eIF4F) complex required for translation (described in the chapter titled Translation). Thus, the translation and decapping machineries compete for the cap. How does deadenylation at the 3′ end of the mRNA render the cap susceptible? Translation is known to involve a physical interaction between bound PABP at the 3′ end and the eIF4F complex at the 5′ end. Release of PABP by deadenylation is thought to destabilize the eIF4F–cap interaction, leaving the cap more frequently exposed. The mechanism is not this simple, though, because additional proteins are known to be involved in the decapping event. A complex of seven related proteins, Lsm1–7, binds to the oligo(A) tract after loss of PABP and is required for decapping. Furthermore, a number of decapping enhancers have been discovered. The mechanisms by which these proteins stimulate decapping are not fully understood, although they appear to act either by recruiting/stimulating the decapping machinery or by inhibiting translation.
In the second pathway (Figure 1, right), deadenylation to oligo(A) is followed by 3′ to 5′ exonuclease digestion of the body of the mRNA. This degradation step is catalyzed by the exosome, a ring-shaped complex consisting of a nine-subunit core with one or more additional proteins attached to its surface. A recent report showed that the exosome also has endonuclease activity, and the function of this activity in mRNA decay remains unknown. The exosome exists in similar form in archaea and is also analogous to the bacterial degradosome in that its core subunits are structurally related to PNPase. Thus, the exosome is an ancient piece of molecular machinery. The exosome also plays an important role in the nucleus, described in the section in this chapter titled Newly Synthesized RNAs Are Checked for Defects via a Nuclear Surveillance System.
The relative importance of each mechanism is not yet known, although in yeast the deadenylation-dependent decapping pathway seems to predominate. The pathways are at least partially redundant. Hundreds of yeast mRNAs were examined by microarray analysis in cells in which either the 5′ to 3′ or 3′ to 5′ pathway was inactivated. In either case, only a small percentage of transcripts increase in abundance relative to wild-type cells. This finding suggests that few yeast mRNAs have a requirement for one or the other pathway. It has been proposed that these deadenylation-dependent pathways represent the default degradation pathways for all polyadenylated mRNAs, though subsets of mRNAs can be targets for other specialized pathways, described in the next section in this chapter titled Other Degradation Pathways Target Specific mRNAs. Even those mRNAs that are degraded by the default pathways, however, are degraded at different mRNA-specific rates.
 الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
الاكثر قراءة في مواضيع عامة في الاحياء الجزيئي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















