


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 1-1-2017
Date: 3-1-2017
Date: 23-6-2019
|
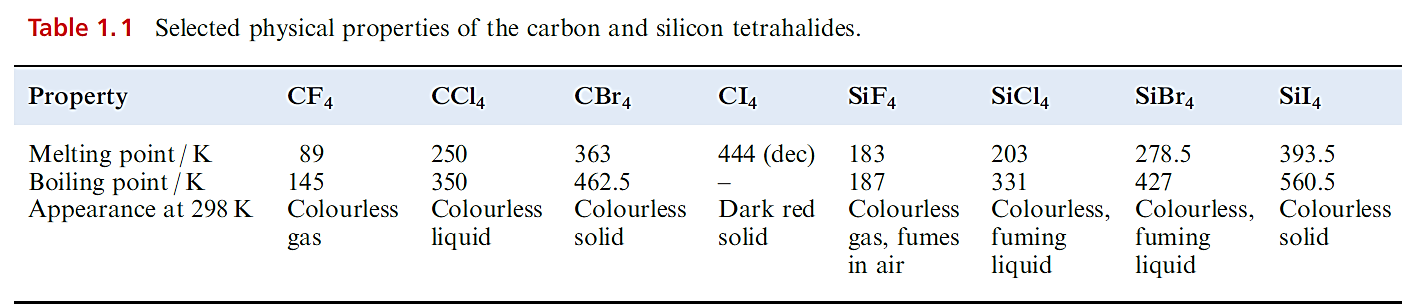
Carbon halides
Selected physical properties of the tetrahalides of C and Si are listed in Table 1.1. The carbon tetrahalides differ markedly from those of the latter group 14 elements: they are inert towards water and dilute alkali and do not form complexes with metal halides. The distinction has been attributed to the absence of d orbitals in the valence shell of a C atom; look back at the electronic versus steric debate. However, one must be cautious. In the case of CX4 being inert towards attack by water, the ‘lack of C d orbitals’ presupposes that the reaction would proceed through a 5-coordinate intermediate (i.e. as is proposed for hydrolysis of silicon halides). Of course, it is impossible to establish the mechanism of a reaction that does not occur! Certainly, CF4 and CCl4 are thermodynamically unstable with respect to hydrolysis; compare the value of ΔrGo with that of ≈ 290 kJ mol-1 for the hydrolysis of SiCl4.
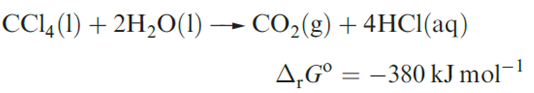
Carbon tetrafluoride is extremely inert and may be prepared by the reaction of SiC and F2, with the second product, SiF4, being removed by passage through aqueous NaOH. Equation 1.16 shows a convenient laboratory-scale synthesis of CF4 from graphite-free calcium cyanamide trace amounts of CsF are added to prevent the formation of NF3.
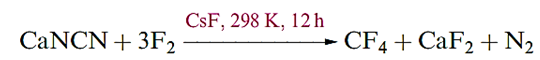
Uncontrolled fluorination of an organic compound usually leads to decomposition because large amounts of heat are evolved (equation 1.17).
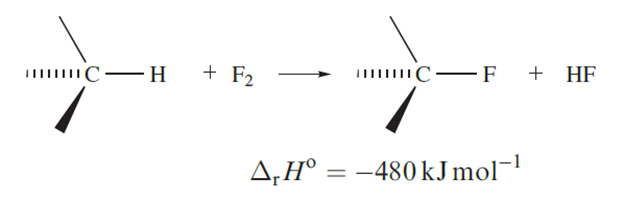
The preparation of a fully fluorinated organic compound tends therefore to be carried out in an inert solvent (the vaporization of which consumes the heat liberated) in a reactor packed with gold- or silver-plated copper turnings (which similarly absorb heat but may also play a catalytic role). Other methods include use of CoF3 or AgF2 as fluorinating agents, or electrolysis in liquid HF.
Fluorocarbons have boiling points close to those of the corresponding hydrocarbons but have higher viscosities. They are inert towards concentrated alkalis and acids, and dissolve only in non-polar organic solvents. Their main applications are as high-temperature lubricants. Although CFCs have been used extensively in aerosol propellants, air-conditioners, foams for furnishings, refrigerants and solvents, concern over their role in the depletion of the ozone layer has resulted in rapid phasing out of their use.
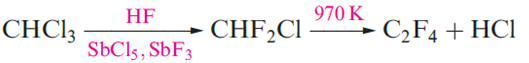
Two important polymers are manufactured from chlorofluoro- compounds. The monomer for the commercially named Teflon or PTFE is C2F4 (tetrafluoroethene) polymerization occurs in the presence of water with an organic peroxide catalyst. Teflon is an inert white solid, stable up to 570 K; it has widespread domestic applications, e.g. non-stick coatings for kitchenware. The monomer CF2=CFCl is used to manufacture the commercial polymer Kel-F. Both Teflon and Kel-F are used in laboratory equipment such as sealing tape and washers, parts in gas cylinder valves and regulators, coatings for stirrer bars, and sleeves for glass joints operating under vacuum. Carbon tetrachloride (Table 1.1) is produced by chlorination of CH4 at 520–670K or by the reaction sequence , in which the CS2 is recycled.



|
|
|
|
دخلت غرفة فنسيت ماذا تريد من داخلها.. خبير يفسر الحالة
|
|
|
|
|
|
|
ثورة طبية.. ابتكار أصغر جهاز لتنظيم ضربات القلب في العالم
|
|
|
|
|
|
|
تضمنت إقامة صلاة العيد واستقبال المهنئين.. مركز دولي تابع للعتبة الحسينية يقيم دورات دينية وجولات تبليغية في إندونيسيا
|
|
|