


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 24-10-2018
Date: 1-4-2019
Date: 6-11-2018
|
Hydroxides of Group 1 metals
In 2002, ≈45Mt of NaOH (caustic soda) were used worldwide, with about one-third of this total being manufactured in the US. NaOH is used throughout organic and inorganic chemistry wherever a cheap alkali is needed. Solid NaOH (mp 591 K) is often handled as flakes or pellets, and dissolves in water with considerable evolution of heat. Potassium hydroxide (mp 633 K) closely resembles NaOH in preparation and properties. It is more soluble than NaOH in EtOH, in which it produces a low concentration of ethoxide ions this gives rise to the use of ethanolic KOH in organic synthesis.

The crystal structures of the group 1 hydroxides are usually complicated, but the high-temperature form of KOH has the NaCl lattice, with the [OH]- ions undergoing rotation rendering them pseudo-spherical. The reactions of alkali metal hydroxides with acids and acidic oxides call for no special mention.

Many non-metals disproportionate when treated with aqueous alkali: P4 gives PH3 and [H2PO2]-, S8 gives S2- and a mixture of oxoanions, and Cl2 reacts to give Cl- and [OCl]- or [ClO3]-. Non-metals that do not form stable hydrides, and amphoteric metals, react with aqueous MOH to yield H2 and oxoanions.
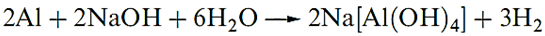



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|