
تاريخ الفيزياء

علماء الفيزياء


الفيزياء الكلاسيكية

الميكانيك

الديناميكا الحرارية


الكهربائية والمغناطيسية

الكهربائية

المغناطيسية

الكهرومغناطيسية


علم البصريات

تاريخ علم البصريات

الضوء

مواضيع عامة في علم البصريات

الصوت


الفيزياء الحديثة


النظرية النسبية

النظرية النسبية الخاصة

النظرية النسبية العامة

مواضيع عامة في النظرية النسبية

ميكانيكا الكم

الفيزياء الذرية

الفيزياء الجزيئية


الفيزياء النووية

مواضيع عامة في الفيزياء النووية

النشاط الاشعاعي


فيزياء الحالة الصلبة

الموصلات

أشباه الموصلات

العوازل

مواضيع عامة في الفيزياء الصلبة

فيزياء الجوامد


الليزر

أنواع الليزر

بعض تطبيقات الليزر

مواضيع عامة في الليزر


علم الفلك

تاريخ وعلماء علم الفلك

الثقوب السوداء


المجموعة الشمسية

الشمس

كوكب عطارد

كوكب الزهرة

كوكب الأرض

كوكب المريخ

كوكب المشتري

كوكب زحل

كوكب أورانوس

كوكب نبتون

كوكب بلوتو

القمر

كواكب ومواضيع اخرى

مواضيع عامة في علم الفلك

النجوم

البلازما

الألكترونيات

خواص المادة


الطاقة البديلة

الطاقة الشمسية

مواضيع عامة في الطاقة البديلة

المد والجزر

فيزياء الجسيمات


الفيزياء والعلوم الأخرى

الفيزياء الكيميائية

الفيزياء الرياضية

الفيزياء الحيوية

الفيزياء العامة


مواضيع عامة في الفيزياء

تجارب فيزيائية

مصطلحات وتعاريف فيزيائية

وحدات القياس الفيزيائية

طرائف الفيزياء

مواضيع اخرى
Radioactive Half-Life
المؤلف:
U.S. Department of Commerce, National Technical Information Service, 1993
المصدر:
The Nuclear Physics and Reactor Theory Handbook
الجزء والصفحة:
p 32
28-3-2017
2052
Radioactive Half-Life
One of the most useful terms for estimating how quickly a nuclide will decay is the radioactive half-life. The radioactive half-life is defined as the amount of time required for the activity to decrease to one-half of its original value. A relationship between the half-life and decay constant can be developed from Equation (1-1). The half-life can be calculated by solving Equation (1-1) for the time, t, when the current activity, A, equals one-half the initial activity Ao .
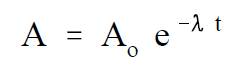 (1-1)
(1-1)
First, solve Equation (1-1) for t.

If A is equal to one-half of Ao , then A/Ao is equal to one-half. Substituting this in the equation above yields an expression for t1/2 .
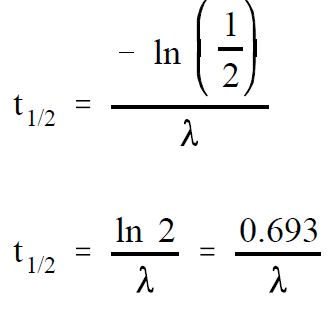 (1-2)
(1-2)
The basic features of decay of a radionuclide sample are shown by the graph in Figure 1.
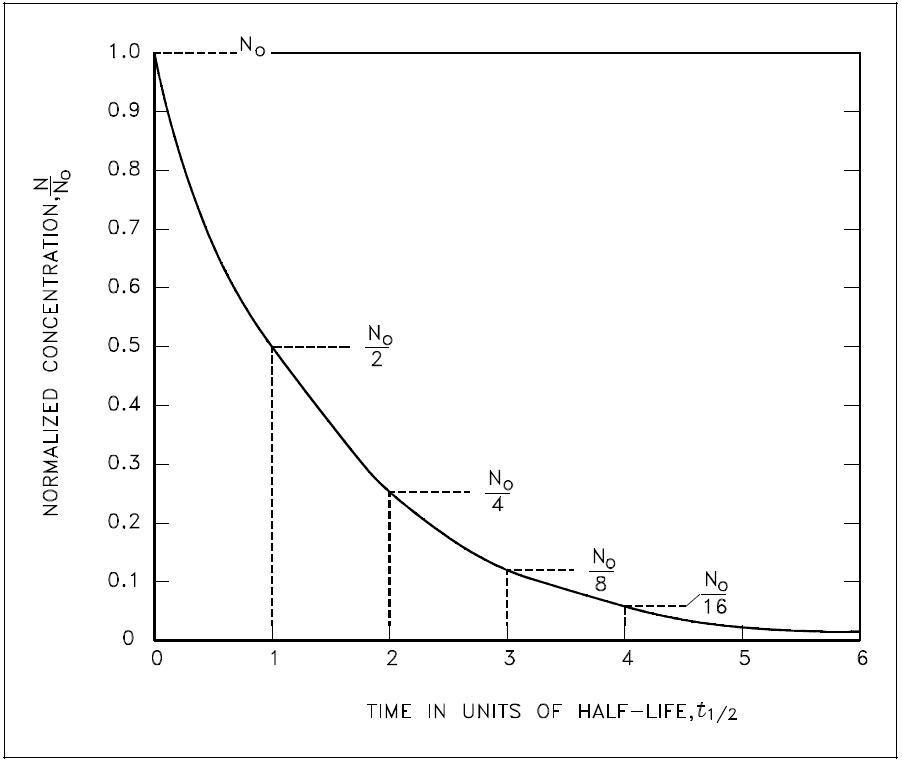
Figure 1: Radioactive Decay as a Function of Time in Units of Half-Life
Assuming an initial number of atoms No, the population, and consequently, the activity may be noted to decrease by one-half of this value in a time of one half-life. Additional decreases occur so that whenever one half-life elapses, the number of atoms drops to one-half of what its value was at the beginning of that time interval. After five half-lives have elapsed, only 1/32, or 3.1%, of the original number of atoms remains. After seven half-lives, only 1/128, or 0.78%, of the atoms remains. The number of atoms existing after 5 to 7 half-lives can usually be assumed to be negligible. The Chemistry Fundamentals Handbook contains additional information on calculating the number of atoms contained within a sample.
 الاكثر قراءة في النشاط الاشعاعي
الاكثر قراءة في النشاط الاشعاعي
 اخر الاخبار
اخر الاخبار
اخبار العتبة العباسية المقدسة

الآخبار الصحية















 قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة
قسم الشؤون الفكرية يصدر كتاباً يوثق تاريخ السدانة في العتبة العباسية المقدسة "المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة
"المهمة".. إصدار قصصي يوثّق القصص الفائزة في مسابقة فتوى الدفاع المقدسة للقصة القصيرة (نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)
(نوافذ).. إصدار أدبي يوثق القصص الفائزة في مسابقة الإمام العسكري (عليه السلام)


















