


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 8-3-2019
Date: 3-1-2017
Date: 5-7-2020
|
Beryllium organometallics
Beryllium alkyls and aryls are best made by reaction types 1.1 and 1.2 respectively. They are hydrolysed by water and inflame in air.
 (1.1)
(1.1)
 (1.2)
(1.2)
In the vapour phase, Me2Be is monomeric, with a linear C_Be_C unit (Be_C = 170pm) . The solid state structure is polymeric (1.1), and resembles that of BeCl2. However, whereas the bonding in BeCl2 can be described in terms of a localized bonding scheme , there are insufficient valence electrons available in (Me2Be)n for an analogous bonding picture. Instead, 3c-2e bonds are invoked as described for BeH2. Higher alkyls are progressively polymerized to a lesser extent, and the tert-butyl derivative is monomeric under all conditions.

(1.1)
 (1.3)
(1.3)
Reaction 1.3 leads to the formation of Cp2Be, and in the solid state, the structure (Figure 1.1a) is in accord with the description (η1-Cp)(η5-Cp)Be. Electron diffraction and spectroscopic studies of Cp2Be in the gas phase have provided conflicting views of the structure, but recent data indicate that it resembles that found in the solid state rather than the (η5-Cp)2Be originally proposed. In solution, however, the 1H NMR spectrum shows that all proton environments are equivalent even at 163 K.

Fig. 1.1 (a) The solid state structure of Cp2Be determined by X-ray diffraction at 128K [K.W. Nugent et al. (1984) Aust. J. Chem., vol. 37, p. 1601]. (b) The same structure showing the two equivalent sites over which the Be atom is disordered. Colour code: Be, yellow; C, grey; H, white.
Furthermore, the solid state structure is not as simple as Figure 1.1a shows; The compound (C5HMe4)2Be can be prepared at room temperature from BeCl2 and K[C5HMe4]. In the solid state at 113 K, it is structurally similar to Cp2Be although, in (C5HMe4)2Be, the Be atom is not disordered. Solution 1H NMR spectroscopic data for (C5HMe4)2Be are consistent with the molecule being fluxional down to 183 K. The fully methylated derivative (C5Me5)2Be is made by reaction 1.4. In contrast to Cp2Be and (C5HMe4)2Be, (C5Me5)2Be possesses a sandwich structure in which the two C5-rings are coparallel and staggered (Figure 1.2), i.e. the compound is formulated as (η5-C5Me5)2Be.
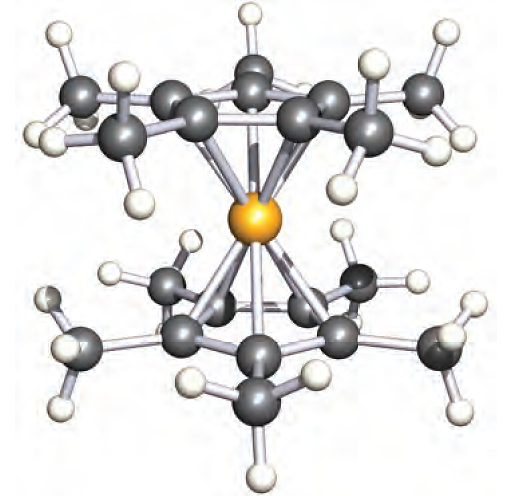
Fig. 1.2 The solid state structure (X-ray diffraction at 113 K) of (η5-C5Me5)2Be [M. del Mar Conejo et al. (2000) Angew. Chem. Int. Ed., vol. 39, p. 1949]. Colour code: Be, yellow; C, grey; H, white.
 (1.4)
(1.4)
In a sandwich complex, the metal centre lies between two π-bonded hydrocarbon (or derivative) ligands. Complexes of the type (η5-Cp)2M are called metallocenes.



|
|
|
|
تفوقت في الاختبار على الجميع.. فاكهة "خارقة" في عالم التغذية
|
|
|
|
|
|
|
أمين عام أوبك: النفط الخام والغاز الطبيعي "هبة من الله"
|
|
|
|
|
|
|
قسم شؤون المعارف ينظم دورة عن آليات عمل الفهارس الفنية للموسوعات والكتب لملاكاته
|
|
|