


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 22-12-2020
Date: 21-12-2020
Date: 21-12-2020
|
Exothermic reactions: Releasing heat
In an exothermic reaction, heat is given off (released) when you go from reactants to products. The reaction between oxygen and methane as you light a gas stove (from the earlier section “Reactants and Products: Reading Chemical Equations”) is a good example of an exothermic reaction.
Even though the reaction gives off heat, you do have to put in a little energy — the activation energy — to get the reaction going. You have to ignite the methane coming out of the burners with a match, lighter, pilot light, or built-in electric igniter.
Imagine that the hypothetical reaction A-B + C → C-A + B is exothermic. The reactants start off at a higher energy state than the products, so energy is released in going from reactants to products. Figure 1.1 shows an energy diagram of this reaction.
In the figure, Ea is the activation energy for the reaction. we show the collision of C and A-B with the breaking of the A-B bond and the forming of the C-A bond at the top of an activation-energy hill. This grouping of reactants at the top of the activationenergy hill is sometimes called the transition state of the reaction.
This transition state shows what bonds are being broken and what bonds are being made. As I show in Figure 1.1, the difference in the energy level of the reactants and the energy level of the products is the amount of energy (heat) that is released in the reaction.
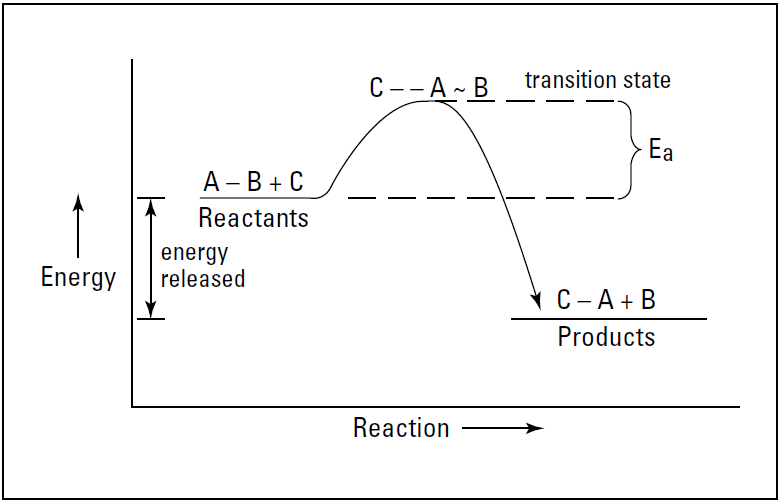
Figure 1.1: Exothermic reaction of A-B + C → C-A + B.



|
|
|
|
كل ما تود معرفته عن أهم فيتامين لسلامة الدماغ والأعصاب
|
|
|
|
|
|
|
ماذا سيحصل للأرض إذا تغير شكل نواتها؟
|
|
|
|
|
|
|
جامعة الكفيل تناقش تحضيراتها لإطلاق مؤتمرها العلمي الدولي السادس
|
|
|