


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 26-2-2019
Date: 3-6-2020
Date: 4-1-2017
|
Getting names from formulas and formulas from names
Sometimes figuring out the charge on an ion can be a little challenging (and fun), so take a look at how to name FeNH4(SO4)2. I show you earlier the sulfate ion has a 2– charge, and from the formula you can see that there are two of these ions. Therefore, you have a total of four negative charges. The ammonium ion has a 1+ charge, so you can figure out the charge on the iron cation:
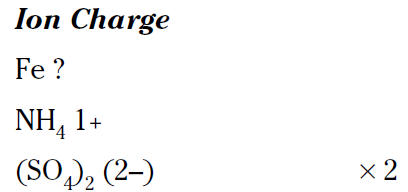
Because you have a 4– charge for the sulfates and a 1+ for the ammonium, the iron must be a 3+ to make the compound neutral. So the iron is in the iron (III), or ferric, oxidation state. You can name the compound:
FeNH4(SO4)2: Iron (III) ammonium sulfate, or ferricammonium sulfate And finally, if you have the name, you can derive the formulaand the charge on the ions. For example, suppose that you’re given the name cuprous oxide. You know that the cuprous ion is Cu+ and the oxide ion is O2–. Applying the crisscross rule (from the earlier section “Using the crisscross rule”), you get the following formula:
Cuprous oxide: Cu2O



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|