


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 31-12-2015
Date: 5-5-2019
Date: 12-2-2018
|
Isomers are different chemical species that have the same chemical formula. Transition metals often form geometric isomers, in which the same atoms are connected through the same types of bonds but with differences in their orientation in space. Coordination complexes with two different ligands in the cis and trans positions from a ligand of interest form isomers. For example, the octahedral [Co(NH3)4Cl2]+ ion has two isomers. In the cis configuration, the two chloride ligands are adjacent to each other. The other isomer, the trans configuration, has the two chloride ligands directly across from one another.
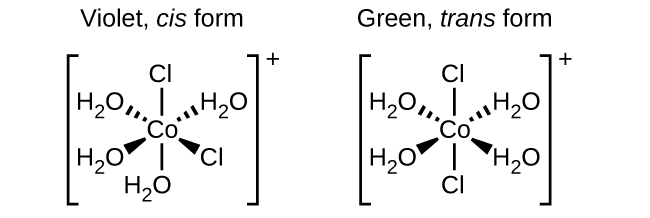
Figure 1 : The cis and trans isomers of [Co(H2O)4Cl2]+ contain the same ligands attached to the same metal ion, but the spatial arrangement causes these two compounds to have very different properties.
Different geometric isomers of a substance are different chemical compounds. They exhibit different properties, even though they have the same formula. For example, the two isomers of [Co(NH3)4Cl2]NO3 differ in color; the cis form is violet, and the trans form is green. Furthermore, these isomers have different dipole moments, solubilities, and reactivities. As an example of how the arrangement in space can influence the molecular properties, consider the polarity of the two [Co(NH3)4Cl2]NO3 isomers. Remember that the polarity of a molecule or ion is determined by the bond dipoles (which are due to the difference in electronegativity of the bonding atoms) and their arrangement in space. In one isomer, cis chloride ligands cause more electron density on one side of the molecule than on the other, making it polar. For the trans isomer, each ligand is directly across from an identical ligand, so the bond dipoles cancel out, and the molecule is nonpolar.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
ندوات وأنشطة قرآنية مختلفة يقيمها المجمَع العلمي في محافظتي النجف وكربلاء
|
|
|