


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
Read More
Date: 6-7-2017
Date: 25-6-2019
Date: 9-2-2018
|
Double bonds: Writing structural formulas for C2H4O
Drawing the structural formula for a molecule that contains a double or triple bond can be a bit tricky. In those cases, your equations may tell you that you have more covalent bonds that you know what to do with. For example, here’s an example of a structural formula that’s a little more complicated C2H4O. The compound has the following framework:
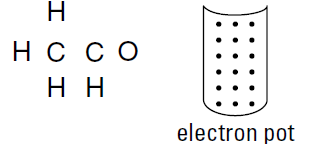
Notice that it has not one but two central atoms — the two carbon atoms. You can put 18 valence electrons into the electron pot: four for each carbon atom, one for each hydrogen atom, and six for the oxygen atom.
Now apply the N – A = S equation:
✓ N = 2(8) + 4(2) + 8 = 32 (two carbon atoms with eight
valence electrons each, plus four hydrogen atoms with two valence electrons each, plus an oxygen atom with eight valence electrons)
✓ A = 2(4) + 4(1) + 6 = 18 (four electrons for each of the two carbon atoms, plus one electron for each of the four hydrogen atoms, plus six electrons for the oxygen atom)
✓ S = 32 – 18 = 14, and S ÷ 2 = 14 ÷ 2 = 7 covalent bonds
Put single bonds between the carbon atoms and the hydrogen atoms, between the two carbon atoms, and between the carbon atom and oxygen atom. That’s six of your seven bonds.
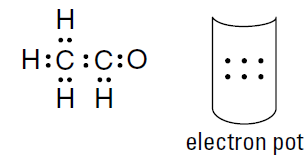
There’s only one place that the seventh bond can go, and that’s between the carbon atom and the oxygen atom. It can’t be between a carbon atom and a hydrogen atom, because that would overfill hydrogen’s valence energy level. And it can’t be between the two carbon atoms, because that would give the carbon on the left ten electrons instead of eight. So there must be a double bond between the carbon atom and the oxygen atom. The four remaining electrons in the pot must be distributed around the oxygen atom, because all the other atoms have reached their octet. Figure 1.1 shows the electrondot formula.
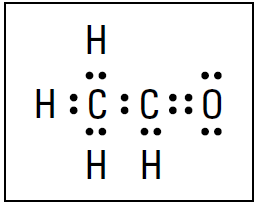
Figure 1.1: Electron-dot formula of C2H4O.
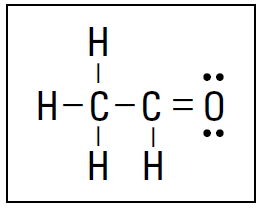
Figure 1.2: The Lewis formula for C2H4O.



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|