


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 20-3-2016
Date: 6-3-2016
Date: 9-3-2016
|
Staphylococcus aureus
1- Introduction
Staphylococcus aureus is a pathogenic bacterium, which causes a foodborne disease classified by the International Commission on Microbiological Specifications for Foods (ICMSF, 2002) in Risk Group III: “diseases of moderate hazard usually not life threatening, normally of short duration without substantial sequelae, with symptoms that are self-limiting but can cause severe discomfort”.
1.1 Taxonomy
1.1.1 The genus Staphylococcus
In the 9th Edition of Bergey’s Manual of Determinative Bacteriology (Holt et al., 1994) the genus Staphylococcus was placed in group 17, which includes the Gram positive cocci. In the 2nd Edition of Bergey’s Manual of Systematic Bacteriology the members of group 17 are subdivided into three phyla: the genus Deinococcus was transferred to the phylum Deinococcus-Thermus, the genus Micro-coccus and the genus Stomatococcus were transferred to the phylum Actinobacteria and the other genera of Gram positive cocci, including Staphylococcus, were transferred to the phylum Firmicutes (Garrity and Holt, 2001).
The affiliation of Micrococcus and Staphylococcus to different phyla indicates a great phylogenetic distance between these genera. However, they share many phenotypic characteristics and in the 1st Edition of Bergey’s Manual of Systematic Bacteriology (Sneath et al., 1986) the two genera were located in the same family.
The phylum Firmicutes includes the Gram positive bacteria with a low DNA mol% G+C content (<50) (Schleifer, 2009). The genus Staphylococcus is a member of the family Staphylococacaeae, which also contains the genera Jeotgalicoccus, Macrococcus and Salinicocus (Schleifer and Bell, 2009a).
The staphylococci cells are spherical and characteristically divide in more than one plane to form irregular grape like clusters. The Gram stain is positive, the cells are nonmotile and non-sporeforming. The catalase reaction is usually positive and oxidase is usually negative. Chemoorganotrophs, the carbohydrate metabolism is respiratory and fermentative. Susceptible to lysis by lysostaphin and resistant to lysis by lysozyme. Predominantly associated with skin, skin glands and mucous membranes of warm-blooded animals (Schleifer and Bell, 2009b).
The Staphylococcus species can be grouped into groups and the most important groups are (Schleifer and Bell, 2009b):
Group S. epidermidis (e.g., S. epidermidis, S. capitis, S. caprae, S. haemolyticus, S. hominis, S. saccharolyticus, S. warneri) and Group S. simulans (e.g., S. simulans, S. carnosos), which are coagulase negative and novobiocin susceptible.
Group S. saprophyticus (e.g., S. saprophyticus, S. cohnii, S. xylosus) and Group S. sciuri (e.g., S. sciuri, S. lentus, S. vitulinus), which are coagulase negative and novobiocin resistant.
Group S. intermedius (e.g., S. intermedius, S. delphini) and Group S. aureus (e.g., S. aureus subsp. aureus, S. aureus subsp. anaerobius), which are coagulase positive and novobiocin susceptible.
1.1.2 The coagulase positive staphylococci
The coagulase positive staphylococci are S. aureus, S. intermedius, S. delphini, and S. schleiferi subsp. coagulans. S. hyicus is coagulase variable. These species are considered potentially serious pathogens (Schleifer and Bell, 2009b) and, for this reason, the production of coagulase is considered as an indication of pathogenicity among the species of Staphylococcus.
According to MacFaddin (2000), coagulase is an enzyme which converts fibrinogen to fibrin, resulting in a visible clot. It may be present in two forms, the bound coagulase or “clumping factor” and the free coagulase or “clotting factor”. The free coagulase is extracellular and reacts with the coagulase-reacting factor “CRF” (a thrombin-like substance in the plasma) to form a coagulase-CRF complex. This complex indirectly converts fibrinogen to fibrin forming a clot. The detection is achieved by the tube coagulase test. The clumping factor is located on the surface of cell walls and forms clots with no involvement of “CRF”. It is not inhibited by antibodies to free coagulase and the detection is achieved by the slide coagulase test.
The main characteristics of the coagulase positive staphylococci are shown in Table 1. According to Schleifer and Bell (2009b) S. aureus subsp. aureus is the most common pathogen among the coagulase positive staphylococci. Some strains produce enterotoxins.
S. aureus subsp. anaerobius is found in abcesses of sheep and is also pathogenic for goats. It produces coagulase but does not produce enterotoxins. S. intermedius is opportunistic pathogenic of dogs, S. hyicus is associated with infections in pigs, skin lesions in cattle and horses, osteomyelitis in poultry and cattle, and occasionally with mastitis in cattle. S. delphini is associated with skin lesions in dolphins. S. schleiferi subsp. coagulans is associated with ear otitis in dogs.
Among the coagulase positive strains S. aureus, S. hyicus and S. intermedius are the species associated with food intoxication outbreaks (Bennett and Hait, 2011).
1.1.3 Staphylococcus aureus
S. aureus is subdivided into two subspecies, S. aureus subsp. aureus and S. aureus subsp. anaerobius. The characteristics differentiating the two subspecies are shown in Table 1.
S. aureus subsp. anaerobius grows microaerobically and anaerobically, but the aerobic growth is weak. It is distinguished from S. aureus subsp. aureus by three characteristics: the lack of pigment and clumping factor, the inability to ferment mannitol anaerobically, and the inability to grow at 45°C. The temperature range for optimal growth is 30–40°C and it does not grow at 20 or 45°C. All strains tolerate 10% of NaCl; most do not tolerate 15%. The primary isolation requires a medium supplemented with serum, egg yolk or blood (Schleifer and Bell, 2009b).
Table.1 Biochemical and growth characteristics of the species and subspecies of coagulase positive Staphylococcus (Schleifer and Bell, 2009).
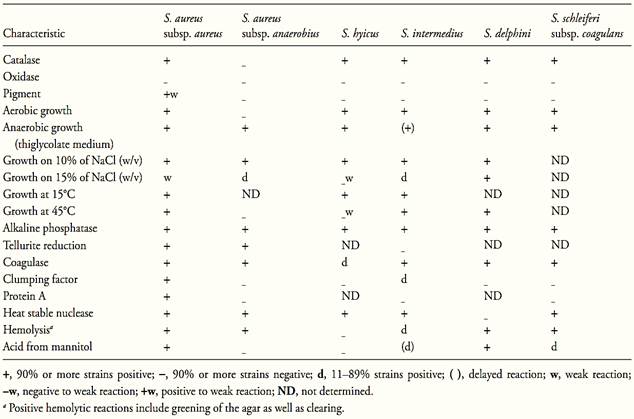
S. aureus subsp. aureus is usually called only S. aureus in the literature, without mention of the subspecies. According to Schleifer and Bell (2009b) it is facultative anaerobic but growth is best under aerobic conditions. Protein A is produced and the positive reactions include alkaline phosphatase, coagulase, clumping factor, heat stable nuclease (thermonuclease), hemolysin, and lipase. Acid is produced aerobically from glucose and mannitol. The temperature range for growth is 10 to 45°C and the optimum is 30 to 37°C.
S. aureus is not heat resistant, and is easily destroyed by pasteurization or by normal cooking. Enterotoxins, on the other hand, are highly heat-resistant and survive heat treatments as severe as those used to sterilize low-acid foods (ICMSF, 1996).
S. aureus is a salt tolerant microorganism and according to Schleifer and Bell (2009b) its growth is good in medium containing 10% of NaCl and poor at 15%. According to ICMSF (1996) it grows at a water activity as low as 0.85 (salt content 25% w/w). From this aspect, S. aureus is an atypical bacterium among the foodborne pathogens, which normally do not grow at such a low water activity.
1.2 Pathogenicity
According to Bien et al. (2011) S. aureus can cause a wide variety of infections, including wound infection, toxinoses (food poisoning, scalded skin syndrome, toxic shock syndrome) and systemic conditions (endocarditis, osteomyelitis, pneumonia, brain abscesses, meningitis, bacteremia).
According to Schleifer and Bell (2009) S. aureus was responsible for considerable morbidity and mortality among hospitalized patients from 1950 to 1960. Methicillin-resistant S. aureus (MRSA) strains emerged in 1980 and become a great epidemiological problem in hospitals. Enterotoxin-producing S. aureus strains are the most common coagulase positive staphylococci associated with food intoxication outbreaks.
1.2.1 Staphylococcus aureus enterotoxins
S. aureus produces various types of toxins. The alpha, beta, delta and gamma toxins, and the leukocidins are involved with cell lysis and tissue invasion (Ferry et al., 2005). The exfoliative toxins (ETs) (also known as “epidermolytic” toxins) are responsible for the staphylococcal scalded skin syndrome (disease characterized by the loss of superficial skin layers, dehydration, and secondary infections) (Bukowski et al., 2010). The toxic shock syndrome toxin (TSST-1) is responsible for the toxic shock syndrome (acute onset illness characterized by fever, rash formation and hypotension that can lead to multiple organ failure and lethal shock (Ferry et al., 2005). The enterotoxins are involved in staphylococcal food poisoning, one of the most common food-borne diseases worldwide.
The enterotoxins responsible for staphylococcal food poisoning are produced primarily by Staphylococcus aureus, although S. intermedius and S. hyicus also have been shown to be enterotoxigenic (Bennett and Hait, 2011). S. intermedius was isolated from butter blend and margarine in a food poisoning outbreak in United States (Khambaty et al., 1994, Bennett, 1996).
A coagulase negative S. epidermidis was reported to have caused at least one outbreak (Breckinridge and Berg-doll, 1971). S. aureus enterotoxins (SEs) and toxic shock syndrome toxin (TSST-1) are broadly classified as super-antigens (SAgs), which have the ability to stimulate large populations of T cells leading to the production of a cytokine bolus (Pinchuk et al., 2010). In 1990 the staphylococcal research community published a standard nomenclature for the superantigens expressed by Staphylococcus aureus (Betley et al., 1990). At this time the classical members of this family included toxic shock–syndrome toxin-1 (TSST-1) and five antigenic variants of S. aureus enterotoxins, designated “SEA”, “SEB”, “SEC”, “SED”, and “SEE” (Lina et al., 2004). The TSST-1 was initially designated as “SEF” (Bergdoll et al., 1981) but was later designated as TSST-1 because did not show in-vivo biological activity characteristic of true enterotoxins (Fueyo et al., 2005).
Newly SEs described after 1990 received a letter designation in the order in which they have been discovered. In 2004 the International Nomenclature Committee for Staphylococcal Superantigens proposed an international procedure for the designation of newly described SAgs and putative SAgs, reported by Lina et al. (2004). The rules are: a) Toxin genes identified but not confirmed to be expressed should not be subject to the standardized toxin nomenclature. b) Only toxins that induce emesis after oral administration in a primate model should be designated as enterotoxin. c) The current letter designation should be retained for SEs in which S = S. aureus and E = enterotoxin. d) The SE should be followed by a letter assigned sequentially until the 25th toxin (SEZ) has been assigned (SEF has been retired). Thereafter, newly described toxins should be numbered sequentially beginning with SE26. e) Related toxins that lack emetic properties (or have not been tested) should be designated “staphylococcal enterotoxin-like” (SEl), to indicate that their potential role in staphylococcal food poisoning has not been confirmed. To minimize confusion and significant renaming, SEls should receive a letter designation in the order in which they are described. If the proteins are later shown to have enterotoxic activities, the SEl designation can be changed to SE. In the IAFP 4th European Symposium on Food Safety a presentation made by Smith (2008) showed the following situation:
• Classic staphylococcal enterotoxins: SEA, SEB, SEC (including three variants SEC1, SEC2, SEC3 and SEC ovine and SEC bovine variants), SED, and SEE.
• Newer SEs: SEG, SEH, SEI, SER, SES, and SET.
• Enterotoxin-like proteins (SEls): SElJ, SElK, SElL, SElM, SElN, SElO, SElP, SElQ, SElU, SElV, SElW.
Only a few of the staphylococcal enterotoxins have been studied in depth. They are pyrogenic and share some other important properties that include the super-antigenicity and the ability to induce emesis and gastro-enteritis (Pinchuk et al., 2010).
The different SE serotypes are identified serologically as separate proteins (Bennett and Hait, 2011). They resist acid and are stable over a wide pH range. They are highly heat-resistant and are not completely denatured by cooking. They are resistant to inactivation by gastrointestinal and other proteases (pepsin, trypsin, chymotrypsin, rennin, papain) (Smith, 2008).
1.2.2 Staphylococcal food poisoning
The disease transmitted by S. aureus is an intoxication, caused by the ingestion of enterotoxins formed in the food as a result of the multiplication of the bacterial cells. The intake of a dose smaller than 1μg may cause symptoms of intoxication and this quantity is reached when the population of S. aureus attains values greater than 105 UFC/g of food (Hait, 2012). The serotype A is the most frequently involved in foodborne staphylococcal illness (Bennett and Hait, 2011).
The symptoms become evident between one to seven hours after ingestion, and include nausea, vomiting, retching and abdominal cramping. Dehydration, headache, muscle cramping, and changes in blood pressure and pulse rate may occur in more severe cases. Recovery occurs in few hours to one day and complications or death are rare (Hait, 2012). It is easily diagnosed, especially in the cause of outbreaks in which nausea and vomiting predominate, and with a short interval between the food ingestion and the onset of symptoms.
S. aureus can be found in the nasal airways, throat, skin and hair of 50% or more of healthy human individuals. Food handlers are a common source of contamination, although equipment and food handling surfaces in processing environments may also contaminate the foods (Hait, 2012).
Foods that have already been implicated in outbreaks include meat and meat products; poultry and egg products; salads, such as egg, tuna, chicken, potato, and macaroni; bakery products, such as cream-filled pastries, cream pies, and chocolate éclairs; sandwich fillings; and milk and dairy products. The foods at greatest risk are those that are intensely handled during their preparation and/or those that remain at room temperature after preparation (Hait, 2012).
1.3 Methods of analysis
There are several methods available for counting S. aureus, the degree of sensitivity of which may vary both in function of the selective/differential characteristics used in formulating the culture media, as in function of the enumeration technique itself (direct plate count or the Most Probable Number technique). Eventually, and depending on the objective of analysis and the conditions of the injuries inflicted on the cells, it may be advisable to use a simple presence/absence test, with a non-selective pre-enrichment step, which will ensure recovery from the injuries.
The main selective characteristics used to isolate S. aureus are the ability of this microorganism to grow in the presence of NaCl (5.5 to 10%), potassium tellurite (0.0025 a 0.05%), lithium chloride (0.01 to 0.05%), glycine (0.12 to 1.26%) and polymyxin (40 mg/l).The differential characteristics are the capacity to reduce potassium tellurite (producing black colonies in solid media), the capacity to hydrolyze egg yolk, the capacity to use mannitol and grow at 42–43°C under selective conditions, the activity of coagulase and the activity of
Table.2 Analytical kits adopted as AOAC Official Methods for Staphylococcus aureus in foods (Horwitz and Latimer, 2010, AOAC International, 2010).

thermonuclease. There are several growth media avail-able for direct plate counts, which combine one or more selective/differential characteristics, such as Mannitol Salt Agar, Vogel-Johnson Agar, Egg Yolk Azide Agar, Phenolphthalein Polymyxin Phosphate Agar, Milk-Salt Agar, Tellurite Glycine Agar, Tellurite Polymyxin Egg Yolk and Staphylococcus Medium number 110. In general, these media should not be used for foods in which the presence of injured cells is expected, since they are considered restrictive to the recovery of injuries. The most widely used medium is Baird-Parker Agar (BP), which combines potassium tellurite (0.01%), glycine (1.2%) and lithium chloride (0.5%), as selective agents and the reduction of tellurite and the hydrolysis of egg yolk as differential characteristics. In addition, the medium contains 1% of sodium pyruvate, which is considered an excellent means to recover injured cells, since it avoids accumulation of hydrogen peroxide (toxic for cells). It may be used for direct plating of processed or fresh (“in natura”) foods, both for enumeration for indicative purposes as for enumeration for public health purposes. On the other hand, BP Agar, as well as all the other media cited above, is not able to completely suppress the growth of competitors of S. aureus. Other non-pathogenic species of the Staphylococcus genus may grow, producing similar colonies, thereby creating the need to subject typical colonies to the coagulase test for confirmation. Other methods that already have been officially recognized by the AOAC International are microbiological test kits described in Table.2.
References
AOAC International (2010). Rapid Methods Adopted as AOAC Official MethodsSM. [Online] Available from: http://www.aoac. org/vmeth/oma_testkits.pdf [Accessed 26th April 2011).
Bennett, R.W. & Hait, J.M. (2011) Staphylococcal enterotoxins: micro-slide double diffusion and elisabased methods. In: FDA (ed.) Bacteriological Analytical Manual, Chapter 13A. [Online] Silver Spring, Food and Drug Administration. Available from: http://www.fda.gov/Food/ScienceResearch/LaboratoryMethods/BacteriologicalAnalyticalManualBAM/default.htm [accessed 10th October 2011].
Bennett, R.W. (1996) Atypical toxigenic Staphylococcus and non-Staphylococcus aureus species on the Horizon? An Update. J. Food Protection, 59, 1123–1126.
Bergdoll, M.S., Crass, B.A., Reiser, R.F., Robbins, R.N. & Davis, J.P. (1981) A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet, 1, 1017–1021.
Betley, M.J., Schlievert, P.M., Bergdoll, M.S., Bohach, G.A., Landolo, J.J., Khan, S.A., Patee, P.A. & Reiser, R.F. (1990). Staphylococcal gene nomenclature. ASM News, 56:182.
Bien, J., Sokolova, O. & Bozko, P. (2011) Characterization of Virulence Factors of Staphylococcus aureus: Novel Function of Known Virulence Factors That Are Implicated in Activation of Airway Epithelial Proinflammatory Response. Journal of Pathogens [Online], 1–13. Available from http://www.hindawi.com/jour-nals/jpath/2011/601905/ [Accessed 14th October 2011].
Bukowski, M., Wladyka, B. & Dubin, G. (2010) Exfoliative toxins of Staphylococcus aureus. Toxins, 2, 1148–1165.
Ferry, T., Perpoint, T., Vandenesch, F. & Etienne, J. (2005) Virulence determinants in Staphylococcus aureus and their involvement in clinical syndromes. Current Infectious Disease Reports, 7, 420–428.
Garrity, G.M. & Holt J.G. (2001) The road map to the Manual. In: Boone, D.R. & Castenhols, R.W. (eds). Bergey’s Manual of Sys-tematic Bacteriology. 2nd edition, Volume 1. New York, Springer. pp. 119–155.
Holt, J.G., Krieg, N.R., Sneath, P.H.A., Staley, J.T. & Williams, S.T. (1994) Bergey’s Manual of Determinative Bacteriology. 9th edition. Baltimore, Williams & Wilkins.
Horwitz, W. & Latimer, G.W. (eds) (2010) Official Methods of Analysis of AOAC International. 18th edition., revision 3. Gaithersburg, Maryland, AOAC International.
ICMSF (International Commission on Microbiological Specifica-tions for Foods) (1996) Microorganisms in Foods 5. Microbiological Specifications of Food Pathogens. London, Blackie Academic & Professional.
Khambaty, F.M.,Bennett, R.W. & Shah, D.B. (1994) Application of pulsed-field gel electrophoresis to the epidemiological characterization of Staphylococcus intermedius implicated in a food-related outbreak. Epidemiology and Infection, 113, 75–81.
Lina, G., Bohach, G.A., Nair, S.P., Hiramatsu, K., Jouvin-Marche, E. & Mariuzza, R. (2004) Standard Nomenclature for the Super-antigens Expressed by Staphylococcus. Journal of Infectious Dis-eases, 189, 2334–2336
MacFaddin, J.F. (2000) Biochemical Tests for Identification of Medical Bacteria. 3rd edition. Philadelphia, Lippincott Williams and Wilkins. pp. 363–367.
Pinchuk, I.V., Beswick, E.J. & Reyes, V.E. (2010) Staphylococcal Enterotoxins. Toxins, 2, 2177–2197.
Schleifer, K & Bell, J.A. (2009a) Family VIII Staphylococcaceae fam. nov. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. p. 392.
Schleifer, K & Bell, J.A. (2009b) Genus I Staphylococcus Rosen-bach 1884. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. pp. 392–421.
Schleifer, K. (2009) Phylum XIII Firmicutes. In: DeVos, P., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A. Schleifer, K. & Whitman, W.B. (eds). Bergey’s Manual of Systematic Bacteriology. 2nd edition, Volume 3. New York, Springer. p. 19.
Silva, N.D .; Taniwaki, M.H. ; Junqueira, V.C.A.; Silveira, N.F.A. , Nasdcimento , M.D.D. and Gomes ,R.A.R .(2013) . Microbiological examination methods of food and water a laboratory Manual. Institute of Food Technology – ITAL, Campinas, SP, Brazil .
Sneath, P.H.A., Mair, N.S., Sharpe, M.E. & Holt, J.G. (eds) (1986) Bergey’s Manual of Systematic Bacteriology. 1st edition, Volume 2. Baltimore, Williams & Wilkins.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|