


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 15-5-2016
Date: 9-5-2021
Date: 8-5-2016
|
Density Gradient Centrifugation
Many centrifugation separations simply centrifuge an initially homogeneous sample and physically remove any heavy and large particles or molecules from the solution, leaving them as a pellet on the bottom of the centrifugation tube. In contrast, density gradient separations employ a gradient of varying, high concentrations of a small molecule, such as sucrose or cesium chloride, distributed along the axis of the centrifugally generated force. The lowest concentration is at the solution meniscus and the highest at the base. With sufficiently high concentrations of the small molecule, the density and viscosity of the solution also vary along the gradient. The density gradient is produced directly before centrifugation, or it is generated by the centrifugal force itself. A wide variety of materials is used to form gradients (Table 1), depending on which components are to be separated.
Table 1. Gradient Materials Used for Density Gradient Centrifugation a
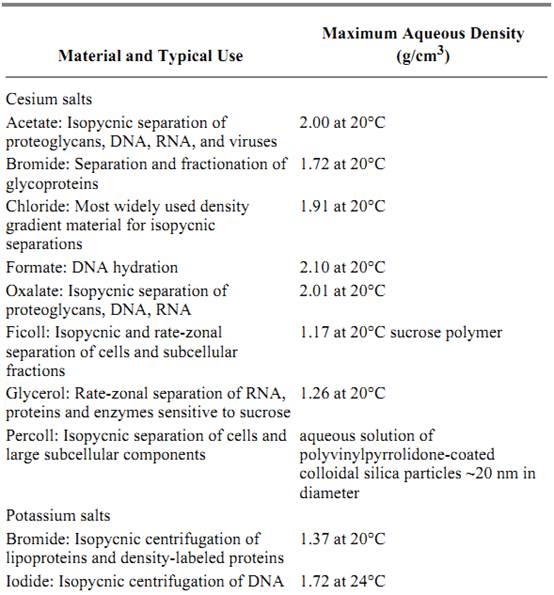

a Adapted from Spinpro Software from Beckman Instruments, a useful software tool. Unfortunately it still runs only under MS-DOS on Intel processor-based computer.
When a sample of molecules is subjected to centrifugation in a density gradient, they sediment toward the bottom if they have a greater buoyant density than the solution, or they will float toward the top if they have a lower density. The rates of sedimentation through the gradient of the various molecules can be measured, as in sedimentation velocity centrifugation, and depends on their buoyant densities and sedimentation coefficients (s-values). Alternatively, the centrifugation can be continued until each molecule reaches that point in the gradient when the solution density is the same as its buoyant density. At this point the molecule neither sediments nor floats. Then different molecules are separated on the basis of differences in their buoyant densities, which can be measured in this way. The physical process of banding significantly improves the resolution of this technique.
1. Rate-Zonal Centrifugation
Separations of macromolecules or subcellular fractions with different sedimentation rates and/or sizes are accomplished by layering the sample of material as a narrow zone onto the top of a preformed gradient of the appropriate material (Table 1) and subjecting the sample to centrifugation. During centrifugation, the constituent components of the sample proceed through the gradient as individual zones, each at its specific sedimentation rate. The sedimentation process results in separating the sample components into discrete bands or zones that can be recovered from the centrifuge tube, typically by simple fraction collection (below). The shape of the gradient, that is, the change in solution density as a function of the physical length of the centrifuge tube or distance from the center of rotation, is a function of the gradient material and its concentration, the rate of centrifugation, etc. Five principal types of gradient systems used in rate-zonal separations are summarized in Table 2
Table 2. Principle Types of Gradients in Rate-Zonal Centrifugation a
a Adapted from Spinpro Software from Beckman Instruments. 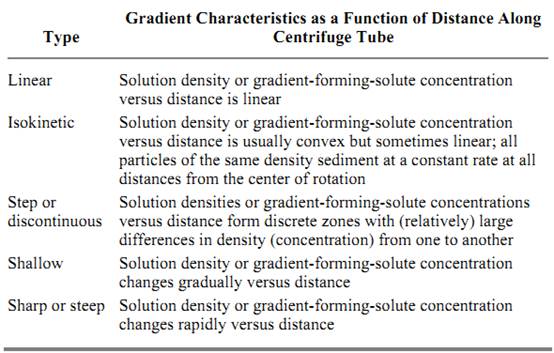
a Adapted from Spinpro Software from Beckman Instruments.
Approximate values of the sedimentation coefficients (s-values) of macromolecules and subcellular components can be obtained from rate-zonal gradient centrifugation without purifing the protein. This is accomplished in isokinetic gradients, where particles sediment at a constant rate, as in sucrose gradient centrifugation. To determine the s-value, it is necessary to include sedimentation “markers” of macromolecules with known sedimentation coefficients. It is necessary only to be able to differentiate between the various molecules, perhaps by their spectroscopic properties or by their biological activities.
2. Flotation in Gradient Centrifugation
This technique is a subclass or offshoot of gradient techniques. It occurs in centrifugation when the buoyant densities of the centrifuged macromolecule or subcellular components are less than that of the solution in which they are centrifuged. The rates of flotation of the individual solution components to the top of the solution are a function of their sizes, shapes and densities, just as in sedimentation to the bottom of the tube.
3. Isopycnic Gradient Centrifugation
Isopycnic procedures are used to separate macromolecules on the basis of their buoyant densities. The density gradient is chosen so as to include the densities of the species to be separated from each other. The sample to be sedimented may be prepared either by homogeneously distributing it throughout the gradient or by layering it on top of the gradient. In either case, when centrifugal force is applied to the sample, the sedimenting components move along the density gradient to where they have the same buoyant density. If they are at a lower solution density initially, they tend to sediment, whereas they tend to float when at a higher solution density. When the density gradient is maintained by the centrifugal force, and perhaps even generated by it, such as with cesium chloride (Table 1), the gradient is “self-generating.” When all the molecules present are at their equilibrium positions, the centrifugal force condenses the molecules into sharp zones and maintains them. Unlike rate-zonal centrifugation, where the zones of the various molecules are constantly broadened by diffusion, but like isoelectric focusing, isopycnic density gradient centrifugation condenses the sample into a narrow zone and keeps it stable and sharp in time. This results from two opposing force fields:
diffusion, which tends to dissipate the zone, and the centrifugal field, which forces any “escaping” molecule back into its appropriate zone.
If the components to be separated have small differences in buoyant densities, the gradient used should bracket the desired density range and should also be shallow, so as to afford significant separation between components. Alternatively, if the components to be separated have large differences in buoyant densities, the gradient can be adjusted so that any undesired component either floats to the top of the gradient or sediments to the bottom, whereas the desired components are within the middle of the gradient.
4. Gradients of Viscous Materials
When viscous gradient materials, such as sucrose, Ficoll, Percoll, and glycerol, are used, the gradients are often prepared by layering steps of decreasing density in the centrifuge tube. Then the various steps are allowed to diffuse into each other (usually for 1 to 3 hours at room temperature) to form an approximately linear gradient. Alternatively, the sample is layered and centrifuged immediately through the series of discontinuous step zones; the sample often accumulates at an interface between two steps, so the steps are chosen so that different materials accumulate at different interfaces. Often a cushioning layer of high density gradient material is incorporated into the bottom of the tube to prevent the desired sample from pelleting onto the tube.
5. Salt Gradients
Cesium chloride is sufficiently heavy that it forms a substantial density gradient under the sole influence of rapid centrifugation. In this case, the starting solution can be uniform in both the cesium chloride and the sample. During centrifugation, the density gradient of cesium chloride is generated, and the molecules of the sample migrate to their isopycnic positions. The appropriate initial cesium chloride concentration must be chosen to give the desired density gradient with the centrifugal force used. It is possible to shorten the time required for attaining equilibrium by preforming the gradient, which then requires centrifugation runs of only 2 to 8 hours. Often a cushioning layer of high density gradient material is incorporated to prevent complete pelleting of the sample on the bottom of the tube.
6. Fractionating Gradients After Centrifugation
The density gradients employed aid the process of recovering the samples, minimizing the disturbance of banded zones in a centrifuge tube. Methods for recovering the banded samples depend on whether the tube in which the experiment has been performed is reusable or disposable. In the former case, the tube is held rigidly while a flat-end syringe needle of appropriate length is carefully inserted at the center of the tube down through the length of the column of gradient solution to the tube base. Then the contents are slowly pumped out. In the latter case, the bottom of the tube is punctured with a needle, and the tube contents are permitted to flow out slowly. Then they are collected in a fraction collector, perhaps after passing through a UV absorbance, fluorescence, or other detector. An alternative fractionation device for this process, a centrifuge tube slicer, is available from Beckman Instruments.
Once separated into individual fractions, the gradient samples can be analyzed for the molecules of interest, such as their enzymatic activity, spectral properties, or a radiolabel. The exact shape of the density gradient at the end of the centrifugation is determined by measuring the refractive index of the solution.
7. Centrifuge Rotors for Gradient Density Separations
Three types of rotor are used for density gradient centrifugation, varying in the orientation of the sample during centrifugation. In the swinging bucket rotor, the sample swings outward away from diffusion, which tends to dissipate the zone, and the centrifugal field, which forces any “escaping” molecule back into its appropriate zone.



|
|
|
|
مخاطر خفية لمكون شائع في مشروبات الطاقة والمكملات الغذائية
|
|
|
|
|
|
|
"آبل" تشغّل نظامها الجديد للذكاء الاصطناعي على أجهزتها
|
|
|
|
|
|
|
تستخدم لأول مرة... مستشفى الإمام زين العابدين (ع) التابع للعتبة الحسينية يعتمد تقنيات حديثة في تثبيت الكسور المعقدة
|
|
|