


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 2024-05-14
التاريخ: 2023-07-11
التاريخ: 1-11-2016
التاريخ: 2024-05-06
|
You first should decide what type of compound it is. The decision usually is straightforward for hydrocarbons, which will fall in one or the other of the categories alkanes, alkenes, alkynes, arenes, cycloalkanes, and so on. But when the compound has more than one functional group it is not always obvious which is the parent function. For example, Compound 1 could be named as an alkene (because of the double-bond function) or as an alcohol (because of the OH function):
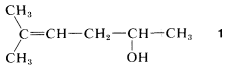
There are no simple rules to follow that dictate which is the parent function, and we suggest that the order of precedence of functional groups set by Chemical Abstracts be used whenever possible (see Table 7-1). By this system, the OH group takes precedence over hydrocarbons, and Compound 11 therefore is named as an alcohol, not as an alkene.
Having decided on the main classification, our next step is to identify the longest chain that includes the main functional group. Then this chain is numbered, starting at the end that gives the main function the lowest possible number. The remaining groups, functional or nonfunctional, are taken as substituents and are assigned numbers according to their position along the chain. Thus for Compound :
1. The longest continuous carbon chain carrying the OH group is a six-carbon unit. The prefix for a six-carbon hydrocarbon is hex-.
2. The chain is numbered so the OH group is at C2, the lowest possible number. Therefore the IUPAC suffix is -2-ol, in which ol signifies alcohol .
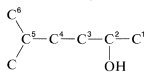
3. The remaining functions are methyl (at C5) and -en(e) (at C4). The complete name is
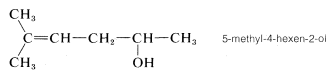
(Notice that the final e is dropped from the suffix -ene when followed by another suffix beginning with a vowel.)
One further point of possible confusion is where to locate the numerical symbol for the main functional group in the name. For instance, if the double bond in 11 were absent, we could name the compound either 5-methylhexan-2-ol or 5-methyl-2-hexanol. The rule is to not divide the name unnecessarily. Thus 5-methyl-2-hexanol would be correct and 5-methylhexan-2-ol would be incorrect:
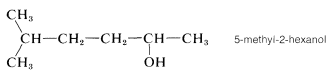



|
|
|
|
دراسة يابانية لتقليل مخاطر أمراض المواليد منخفضي الوزن
|
|
|
|
|
|
|
اكتشاف أكبر مرجان في العالم قبالة سواحل جزر سليمان
|
|
|
|
|
|
|
المجمع العلمي ينظّم ندوة حوارية حول مفهوم العولمة الرقمية في بابل
|
|
|