


 علم الكيمياء
علم الكيمياء 
 الكيمياء التحليلية
الكيمياء التحليلية 
 الكيمياء الحياتية
الكيمياء الحياتية 
 الكيمياء العضوية
الكيمياء العضوية 
 الكيمياء الفيزيائية
الكيمياء الفيزيائية
 الكيمياء اللاعضوية
الكيمياء اللاعضوية 
 مواضيع اخرى في الكيمياء
مواضيع اخرى في الكيمياء
 الكيمياء الصناعية
الكيمياء الصناعية |
أقرأ أيضاً
التاريخ: 2023-08-24
التاريخ: 2024-01-15
التاريخ: 3-4-2017
التاريخ: 20-7-2018
|
We have seen examples of molecules with one chiral center that exist in two mirror-image configurations, which we call enantiomers. What happens when there is more than one chiral center? How many stereoisomers should we expect? Consider the stereoisomers of the important amino acid, threonine, (2-amino-3-hydroxybutanoic acid). For this substance, if we write all of the possible configurations of its two chiral carbons, we have four different projection formulas, 1919-2222, corresponding to four different stereoisomers:
Figure 5-12) and by putting the model over 20-22 verify that none of these projections have the configurations of both chiral carbons the same as your model of 19.
Figure 5-12, except that configurations at each of two atoms are involved. Next, the model is turned and rotated around the center C−C bond to put the methyl and carboxyl groups anti to one another. The Newman formula is then drawn to correspond to the ball-and-stick model.
Because each chiral center added to a chain doubles the number of possible configurations, we expect eight different stereoisomers with three chiral carbons, sixteen with four, and so on, the simple rule the is 2n possible different stereoisomers for nn chiral centers. As we shall see later, this rule has to be modified in some special cases.
What is the relationship between stereoisomers 19-22? This will be clearer if we translate each of the projection formulas into a three-dimensional representation, as shown in Figure 5-13. You will be helped greatly if you work through the sequence yourself with a ball-and-stick model. Drawn as Newman projections, 19-22 come out as shown in 19a-22a:
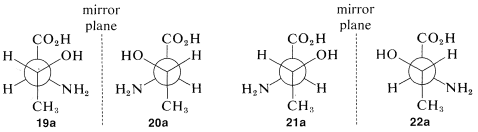
It should be clear (and, if it isn't, ball-and-stick models will be invaluable) that 19a and (20a) are mirror images of one another and that 21a and 22a are similarly mirror images. What about other combinations such as 19a and 21a or 20a and 22a? If you look at the pairs closely you will find that they are not mirror images and are not identical. Such substances, related to each other in this way and which can be converted one into the other only by changing the configurations at one or more chiral centers, are called diastereomers.
The difference between enantiomers and diastereomers is more than just geometry. Diastereomers have substantially different chemical and physical properties, whereas enantiomers have identical physical properties (apart from their optical rotations). This is illustrated in Table 5-1 for the threonine stereoisomers. The reason for the difference in physical properties between diastereomers can be seen very simply for a substance with two chiral centers by noting that a right shoe on a right foot (D,D) is a mirror image, or has the same physical properties, as a left shoe on a left foot (L,L), but is not a mirror image, nor does it have the same physical properties, as a left show on a right foot (L,D), or a right shoe on a left foot (D,L).



|
|
|
|
"عادة ليلية" قد تكون المفتاح للوقاية من الخرف
|
|
|
|
|
|
|
ممتص الصدمات: طريقة عمله وأهميته وأبرز علامات تلفه
|
|
|
|
|
|
|
المجمع العلمي للقرآن الكريم يقيم جلسة حوارية لطلبة جامعة الكوفة
|
|
|