

 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 22-11-2021
Date: 2-11-2021
Date: 12-11-2021
|
Electron Transport Chain
Energy-rich molecules, such as glucose, are metabolized by a series of oxidation reactions ultimately yielding carbon dioxide and water (H2O), as shown in Figure 1. The metabolic intermediates of these reactions donate electrons to specific coenzymes, nicotinamide adenine dinucleotide (NAD+) and flavin adenine dinucleotide (FAD), to form the energy-rich reduced forms, NADH and FADH2. These reduced coenzymes can, in turn, each donate a pair of electrons to a specialized set of electron carriers, collectively called the electron transport chain (ETC), described in this section. As electrons are passed down the ETC, they lose much of their free energy. This energy is used to move H+ across the inner mitochondrial membrane, creating a H+ gradient that drives the production of ATP from ADP and inorganic phosphate (Pi), . The coupling of electron transport with ATP synthesis is called oxidative phosphorylation, sometimes denoted as OXPHOS. It proceeds continuously in all tissues that contain mitochondria. [Note: The free energy not trapped as ATP is used to drive ancillary reactions such as transport of calcium ions into mitochondria and to generate heat.]
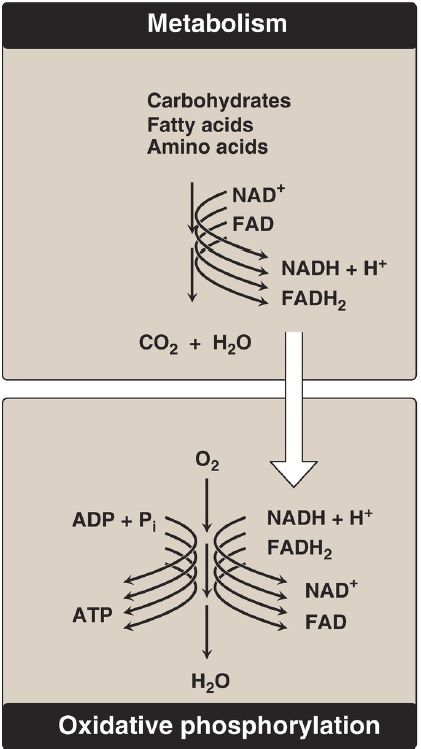
Figure 1: The metabolic breakdown of energy-yielding molecules. NAD(H) =nicotinamide adenine dinucleotide; FAD(H2) = flavin adenine dinucleotide; ADP = adenosine diphosphate; Pi = inorganic phosphate; CO2 = carbon dioxide.
A. Mitochondrial electron transport chain
The ETC (except for cytochrome ) is located in the inner mitochondrial membrane and is the final common pathway by which electrons derived from different fuels of the body flow to oxygen (O2), reducing it to H2O (see Fig. 1).
1. Mitochondrial membranes: The mitochondrion contains an outer and an inner membrane separated by the intermembrane space. Although the outer membrane contains special channels (formed by the protein porin), making it freely permeable to most ions and small molecules, the inner membrane is a specialized structure that is impermeable to most small ions, including H+, and small molecules such as ATP, ADP, pyruvate, and other metabolites important to mitochondrial function (Fig. 2).
Specialized carriers or transport systems are required to move ions or molecules across this membrane. The inner mitochondrial membrane is unusually rich in proteins, over half of which are directly involved in oxidative phosphorylation. It also contains convolutions, called cristae, which greatly increase its surface area.
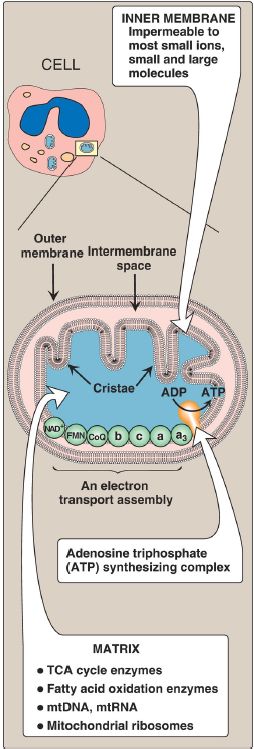
Figure 2: Structure of a mitochondrion showing schematic representation of the electron transport chain and the ATP synthesizing complex on the inner membrane. [Note: Unlike the inner membrane, the outer membrane is highly permeable, and the milieu of the intermembrane space is like that of the cytosol.] mt = mitochondrial; RNA = ribonucleic acid; ADP = adenosine diphosphate; TCA = tricarboxylic acid. 2. Mitochondrial matrix: The gel-like solution of the matrix (interior) of mitochondria is also rich in proteins. These include the enzymes responsible for the oxidation of pyruvate, amino acids, and fatty acids (by β-oxidation) as well as those of the tricarboxylic acid (TCA) cycle.
The synthesis of glucose, urea, and heme occurs partially in the matrix of mitochondria. In addition, the matrix contains NAD+ and FAD (the oxidized forms of the two coenzymes that are required as electron acceptors), and ADP and Pi, which are used to produce ATP. [Note: The matrix also contains mitochondrial deoxyribonucleic acid (mtDNA), ribonucleic acid (mtRNA), and ribosomes.]
B. Organization
The inner mitochondrial membrane contains four separate protein complexes, called Complexes I, II, III, and IV that each contain part of the ETC (Fig. 3). These complexes accept or donate electrons to the relatively mobile electron carrier coenzyme Q (CoQ) and cytochrome c. Each carrier in the ETC can receive electrons from an electron donor and can subsequently donate electrons to the next acceptor in the chain. The electrons ultimately combine with O2 and H+ to form H2O. This requirement for O2 makes the electron transport process the respiratory chain, which accounts for the greatest portion of the body’s use of O2.
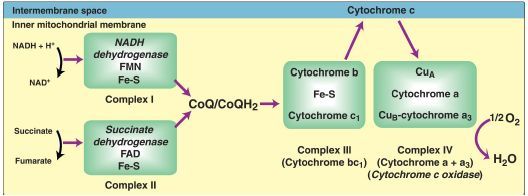
Figure 3: Electron transport chain. Electron flow is shown by magenta arrows. NAD(H) = nicotinamide adenine dinucleotide; FMN = flavin mononucleotide; FAD = flavin adenine dinucleotide; Fe-S = iron-sulfur; CoQ = coenzyme Q; Cu = copper.
C. Reactions
With the exception of CoQ, which is a lipid-soluble quinone, all members of the ETC are proteins. These may function as enzymes as is the case with the flavin-containing dehydrogenases, may contain iron as part of an ironsulfur (Fe-S) center, may contain iron as part of the porphyrin prosthetic group of heme as in the cytochromes, or may contain copper (Cu) as does the cytochrome a + a3 complex.
1. NADH formation: NAD+ is reduced to NADH by dehydrogenases that remove two hydrogen atoms from their substrate. [Note: For examples of these reactions, see the discussion of the dehydrogenases of the TCA cycle] Both electrons but only one H+ (that is, a hydride ion [:H−]) are transferred to the NAD+, forming NADH plus a free H+.
2. NADH dehydrogenase: The free H+ plus the hydride ion carried by NADH are transferred to NADH dehydrogenase, a protein complex (Complex I) embedded in the inner mitochondrial membrane. Complex I has a tightly bound molecule of flavin mononucleotide (FMN), a coenzyme structurally related to FAD that accepts the two hydrogen atoms (2 electrons + 2 H+), becoming FMNH2. NADH dehydrogenase also contains peptide subunits with Fe-S centers (Fig. 4). At Complex I, electrons move from NADH to FMN to the iron of the Fe-S centers and then to CoQ. As electrons flow, they lose energy. This energy is used to pump four H+ across the inner mitochondrial membrane, from the matrix to the intermembrane space.
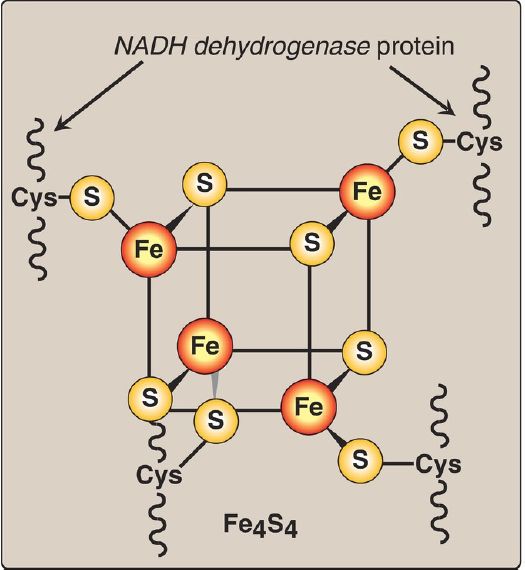
Figure 4:Iron-sulfur (Fe-S) center of Complex I. [Note: Complexes II and III also contain Fe-S centers.] NADH = nicotinamide adenine dinucleotide; Cys =cysteine.
3. Succinate dehydrogenase: At Complex II, electrons from the succinate dehydrogenase–catalyzed oxidation of succinate to fumarate move from the coenzyme, FADH2, to an Fe-S protein, and then to CoQ. [Note: Because no energy is lost in this process, no H+ are pumped at Complex II.]
4. Coenzyme Q: CoQ is a quinone derivative with a long, hydrophobic isoprenoid tail. It is made from an intermediate of cholesterol synthesis . [Note: It is also called ubiquinone because it is ubiquitous in biologic systems.] CoQ is a mobile electron carrier and can accept electrons from NADH dehydrogenase (Complex I), from succinate dehydrogenase (Complex II) and from other mitochondrial dehydrogenases, such as glycerol 3-phosphate dehydrogenase and acyl CoA dehydrogenases (see p. 192). CoQ transfers electrons to Complex III (cytochrome bc1). Thus, a function of CoQ is to link the flavoprotein dehydrogenases to the cytochromes.
5. Cytochromes: The remaining members of the ETC are cytochrome proteins. Each contains a heme group (a porphyrin ring plus iron). Unlike the heme groups of hemoglobin, the cytochrome iron is reversibly converted from its ferric (Fe3+) to its ferrous (Fe2+) form as a normal part of its function as an acceptor and donor of electrons. Electrons are passed along the chain from cytochrome bc1 (Complex III), to cytochrome c, and then to cytochromes a + a3 ([Complex IV] ). As electrons flow, four H+ are pumped across the inner mitochondrial membrane at Complex III and two at Complex IV. [Note: Cytochrome c is located in the intermembrane space, loosely associated with the outer face of the inner membrane. As seen with CoQ, cytochrome c is a mobile electron carrier.]
6. Cytochrome a + a3: Because this cytochrome complex (Complex IV) is the only electron carrier in which the heme iron has an available coordination site that can react directly with O2, it also is called cytochrome c oxidase. At Complex IV, the transported electrons, O2, and free H+ are brought together, and O2 is reduced to H2O. [Note: Four electrons are required to reduce one molecule of O2 to two molecules of H2O.] Cytochrome c oxidase contains Cu atoms that are required for this complicated reaction to occur. Electrons move from CuA to cytochrome a to cytochrome a3 (in association with CuB) to O2.
7. Site-specific inhibitors: Inhibitors of specific sites in the ETC have been identified and are illustrated in Figure 5. These respiratory inhibitors prevent the passage of electrons by binding to a component of the chain, blocking the oxidation-reduction reaction. Therefore, all electron carriers before the block are fully reduced, whereas those located after the block are oxidized. [Note: Inhibition of the ETC inhibits ATP synthesis because these processes are tightly coupled .

Figure 5: Site-specific inhibitors of electron transport shown using a mechanical model for the coupling of oxidation-reduction reactions. [Note: Normal direction of electron flow is illustrated.] NAD+ = nicotinamide adenine dinucleotide; FMN = flavin mononucleotide; CoQ = coenzyme Q; Cyto = cytochrome; CN− = cyanide; CO = carbon monoxide; H2S = hydrogen sulfide; NaN3 = sodium azide.
Leakage of electrons from the ETC produces reactive oxygen species (ROS), such as superoxide (O2 −·), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·). ROS damage DNA and proteins and cause lipid peroxidation. Enzymes such as superoxide dismutase (SOD), catalase, and glutathione peroxidase are cellular defenses against ROS.
D. Free energy release during electron transport
The free energy released as electrons are transferred along the ETC from an electron donor (reducing agent or reductant) to an electron acceptor (oxidizing agent or oxidant) is used to pump H+ at Complexes I, III, and IV. [Note: The electrons can be transferred as hydride ions to NAD+; as hydrogen atoms to FMN, CoQ, and FAD; or as electrons to cytochromes.] 1. Redox pairs: Oxidation (loss of electrons) of one substance is always accompanied by reduction (gain of electrons) of a second. For example, Figure 6 shows the oxidation of NADH to NAD+ by NADH dehydrogenase at Complex I, accompanied by the reduction of FMN, the prosthetic group, to FMNH2. Such redox reactions can be written as the sum of two separate half reactions, one an oxidation and the other a reduction (see Fig. 6). NAD+ and NADH form a redox pair, as do FMN and FMNH2. Redox pairs differ in their tendency to lose electrons. This tendency is a characteristic of a particular redox pair and can be quantitatively specified by a constant, E0 (the standard reduction potential), with units in volts.

Figure 6: Oxidation of NADH by FMN, separated into two component half reactions. NAD(H) = nicotinamide adenine dinucleotide; FMN(H2) = flavin mononucleotide; e− = electron; H+ = proton; E0 = standard reduction potential.
2. Standard reduction potential: The E0 of various redox pairs can be ordered from the most negative E0 to the most positive. The more negative the E0 of a redox pair, the greater the tendency of the reductant member of that pair to lose electrons. The more positive the E0, the greater the tendency of the oxidant member of that pair to accept electrons. Therefore, electrons flow from the pair with the more negative E0 to that with the more positive E0. The E0 values for some members of the ETC are shown in Figure 6. [Note: The components of the chain are arranged in order of increasingly positive E0 values.]
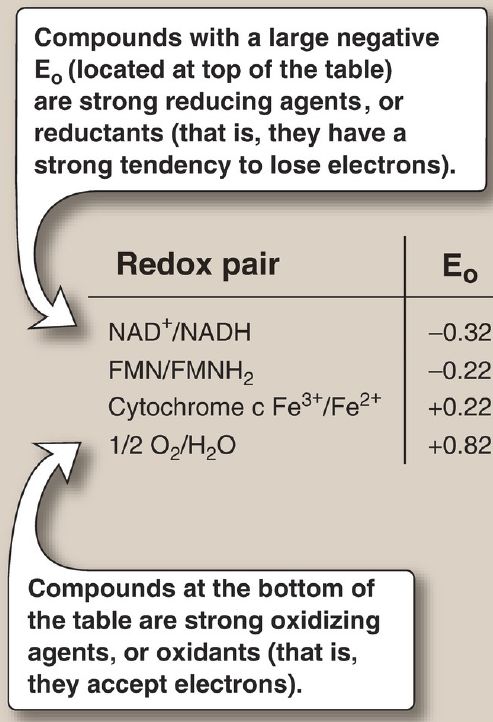
Figure 6: Standard reduction potentials (E0) of some reactions. NAD(H) =nicotinamide adenine dinucleotide; FMN(H2) = flavin mononucleotide; Fe =iron.
3. Relationship of ΔG0 to ΔE0: The ΔG0 is related directly to the magnitude of the change in E0:
where n = number of electrons transferred (1 for a cytochrome, 2 for NADH,
FADH2, and CoQ)
F = Faraday constant (23.1 kcal/volt mol)
ΔE0 = E0 of the electron-accepting pair minus the E0 of the electrondonating
pair
ΔG0 = change in the standard free energy
4. ΔG0 of ATP: The ΔG0 for the phosphorylation of ADP to ATP is +7.3 kcal/mol. The transport of a pair of electrons from NADH to O2 through the ETC releases 52.6 kcal. Therefore, more than sufficient energy is available to produce three ATP from three ADP and three Pi (3 × 7.3 = 21.9 kcal/mol), sometimes expressed as a P/O ratio (ATP made per O atom reduced) of 3:1. The remaining calories are used for ancillary reactions or released as heat. [Note: The P:O for FADH2 is 2:1 because Complex I is bypassed.]



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|