


 النبات
النبات
 الحيوان
الحيوان
 الأحياء المجهرية
الأحياء المجهرية
 علم الأمراض
علم الأمراض
 التقانة الإحيائية
التقانة الإحيائية
 التقنية الحيوية المكروبية
التقنية الحيوية المكروبية
 التقنية الحياتية النانوية
التقنية الحياتية النانوية
 علم الأجنة
علم الأجنة
 الأحياء الجزيئي
الأحياء الجزيئي
 علم وظائف الأعضاء
علم وظائف الأعضاء
 الغدد
الغدد
 المضادات الحيوية
المضادات الحيوية|
Read More
Date: 5-9-2021
Date: 8-10-2021
Date: 6-9-2021
|
Enzyme Properties
Enzymes are protein catalysts that increase the velocity of a chemical reaction and are not consumed during the reaction. [Note: Some ribonucleic acids (RNA) can catalyze reactions that affect phosphodiester and peptide bonds. RNAs with catalytic activity are called ribozymes and are much less common than protein catalysts.]
A. Active site
Enzyme molecules contain a special pocket or cleft called the active site. The active site, formed by folding of the protein, contains amino acid side chains that participate in substrate binding and catalysis (Fig. 1). The substrate binds the enzyme noncovalently, forming an enzyme–substrate (ES) complex. Binding is thought to cause a conformational change in the enzyme (induced fit model) that allows catalysis. ES is converted to an enzyme–product (EP) complex that subsequently dissociates to enzyme and product.
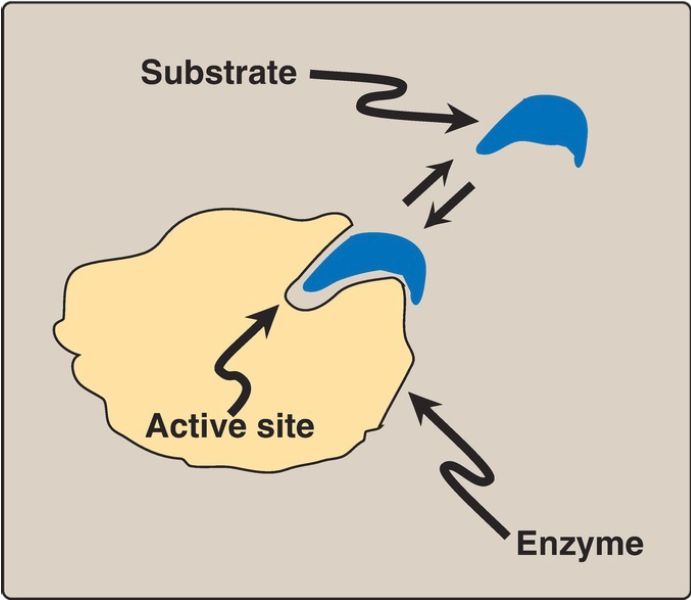
Figure 1: Schematic representation of an enzyme with one active site binding a substrate molecule.
B. Efficiency
Enzyme-catalyzed reactions are highly efficient, proceeding from 103 to 108 times faster than uncatalyzed reactions. The number of substrate molecules converted to product per enzyme molecule per second is called the turnover number, or kcat, and typically is 102–104 s−1. [Note: kcat is the rate constant for the conversion of ES to E + P (see p. 58).]
C. Specificity
Enzymes are highly specific, interacting with one or a few substrates and catalyzing only one type of chemical reaction. The set of enzymes made in a cell determines which reactions occur in that cell.
D. Holoenzymes, apoenzymes, cofactors, and coenzymes
Some enzymes require nonproteins for enzymic activity. The term holoenzyme refers to the active enzyme with its nonprotein component, whereas the enzyme without its nonprotein moiety is termed an apoenzyme and is inactive. If the nonprotein moiety is a metal ion, such as zinc (Zn2+) or iron (Fe2+), it is called a cofactor . If it is a small organic molecule, it is termed a coenzyme. Coenzymes that only transiently associate with the enzyme are called cosubstrates. Cosubstrates dissociate from the enzyme in an altered state (NAD+ is an example). If the coenzyme is permanently associated with the enzyme and returned to its original form, it is called a prosthetic group (FAD is an example). Coenzymes commonly are derived from vitamins. For example, NAD+ contains niacin, and FAD contains riboflavin .
E. Regulation
Enzyme activity can be regulated, that is, increased or decreased, so that the rate of product formation responds to cellular need.
F. Location within the cell
Many enzymes are localized in specific organelles within the cell (Fig. 2). Such compartmentalization serves to isolate the reaction substrate or product from other competing reactions. This provides a favorable environment for the reaction and organizes the thousands of enzymes present in the cell into purposeful pathways.

Figure 2: The intracellular location of some important biochemical pathways. TCA = tricarboxylic acid; PP = pentose phosphate.



|
|
|
|
التوتر والسرطان.. علماء يحذرون من "صلة خطيرة"
|
|
|
|
|
|
|
مرآة السيارة: مدى دقة عكسها للصورة الصحيحة
|
|
|
|
|
|
|
نحو شراكة وطنية متكاملة.. الأمين العام للعتبة الحسينية يبحث مع وكيل وزارة الخارجية آفاق التعاون المؤسسي
|
|
|